Thanks to the success of GLP-1 agonists such as liraglutide and semaglutide in regulating appetite and body weight, incretins have become a field of great interest. Today, incretins as hormones are not only studied by endocrinologists and pharmacists but also by other medical specialties such as gynaecologists, neurologists, and cardiologists, as well as nutritionists and physiotherapists. Their effectiveness fascinates the entire healthcare sector, which has recognized their role in addressing one of the biggest problems in modern society – excessive body weight. A review of current literature gives the impression that modern humans, whose lives are characterized by the intake of energy-rich food and reduced expenditure due to inactivity, are relatively deficient in GLP-1. In other words, the amounts of endogenously produced GLP-1 are not sufficient to adequately regulate both appetite and glucose metabolism in the body, given their diet and physical activity. But are drugs the only way to increase GLP-1 levels in the body? Are there any natural and less harmful alternatives?
The regulation of appetite at the central level is closely linked to the conditions within the gut.15 Recent discoveries have highlighted the significant role of the gut microbiome in regulating appetite through the gut-brain axis. This has sparked interest in developing gut microbiome-targeted therapies for managing appetite. Probiotics, in particular, have emerged as promising tools for modulating gut microbiota composition and function.8 Could therefore probiotics potentially regulate appetite by influencing GLP-1 levels?
Appetite and appetite regulation
Appetite is the neurological impulse that drives us to eat. It varies in intensity, signalling us when to eat or stop eating based on feelings of hunger and satiety. When we feel full, our appetite levels are low; when we feel hungry, our appetite increases.1
The global rise in obesity and an ageing population means more people are experiencing appetite dysregulation. This has led the scientific community to focus on understanding the molecular mechanisms of appetite regulation, aiming to develop potential treatments for these conditions.4
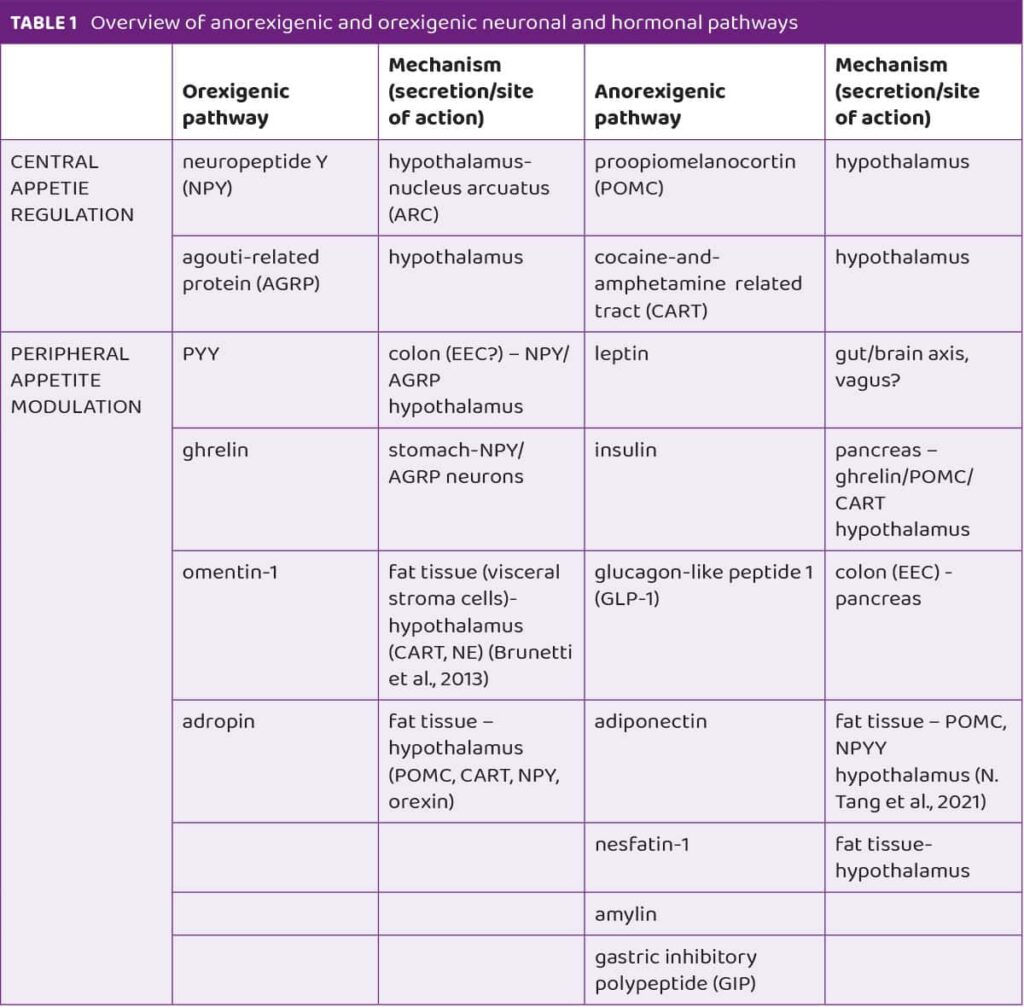
Appetite regulation is a complex process involving neurohormonal and metabolic signals. It depends on the balance between compounds that stimulate appetite (orexigenic) and those that suppress it (anorexigenic).4 These signals interact within the central nervous system and the digestive tract through neuropeptide hormones.
Key hormones and compounds in appetite regulation include leptin, insulin, GLP-1, amylin, ghrelin, omentin-1, nesfatin-1 and adropin. Leptin is regarded as a master regulator of appetite. In obese people visceral adiposity is associated with increased levels of leptin.5 It is interesting that levels of ghrelin, also called the “hunger hormone” rise before meals and decrease after meals hence it has been associated with meal initiation and food intake.6 Another novel appetite-modulating compound is nesfatin-1 which is mainly produced by a subcutaneous fat tissue. Nesfatin-1 regulates appetite and body weight as an anorexigenic adipokine and its levels are increased in obesity and decreased by food deprivation.7 In Table 1 anorexigenic and orexigenic neuronal and hormonal pathways are presented. Understanding these pathways is crucial for exploring how probiotics might influence appetite. The mechanisms of appetite regulation are intricate and rely on a delicate balance of various signals and hormones. Considering that, probiotics may play a role in this process by interacting with these pathways, potentially offering new avenues for managing appetite-related disorders.
Probiotics and appetite
Beyond these benefits, probiotics are known for their anti-inflammatory, pain-relieving, and antioxidant effects. Interestingly, emerging research suggests that probiotics may also have appetite-suppressing (anorexigenic) effects.
Supplementing with probiotics alters the gut microbiota composition, which in turn influences molecules that regulate appetite and satiety. This connection has led to increased interest in developing next-generation, or precision, probiotics. These advanced probiotics are designed to have specific, targeted effects, particularly by producing metabolites that influence the gut-brain axis.14
While the potential of probiotics is promising, there is still a gap in knowledge regarding their anorexigenic effects.
How to probiotics regulate appetite? Probiotics have emerged as promising agents for modulating appetite and weight management through a variety of mechanisms.

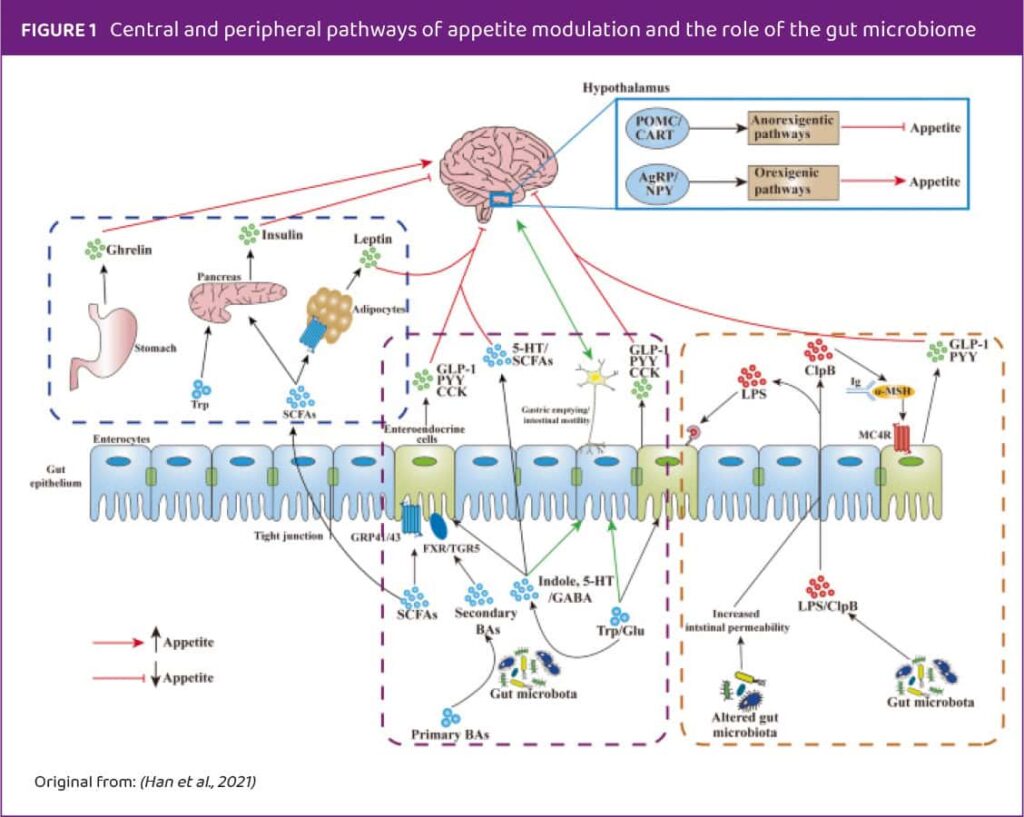
Neurohormonal compounds, such as cholecystokinin (CCK), glucagon-like peptide 1 (GLP-1), and peptide YY (PYY), are produced by enteroendocrine cells (EECs) in the gut. These intestinal hormones play a crucial role in both the digestive system and the central nervous system, influencing various brain regions involved in appetite regulation.
The gut microbiome is the one that interacts closely with these EECs (Figure 1). EECs have nutrient-sensing receptors that detect available nutrients and the composition of the gut microbiome, prompting the release of specific neurohormonal compounds.
Current studies
In order to investigate this effect, various studies on both animals and humans have been performed.
Animal studies have confirmed that the gut microbiome plays a crucial role in the physiological modulation of hunger and satiety regulating various metabolic pathways. As probiotics target the gut microbiome, they can influence this process in several ways. They can alter the gut microbiome composition, change the metabolite profile, and secrete metabolites like short-chain fatty acids (SCFAs), which impact metabolic pathways related to appetite regulation. Probiotics exert their regulatory action on both the peripheral and central levels of appetite modulation. Additionally, by affecting gut microbiome alterations, they can influence digestion and nutrient absorption, further impacting appetite regulation.
A study from Falcinelli et al. demonstrated that administering Lactobacillus rhamnosus at 106 CFU/mL to 6-day-old zebrafish larvae changed the gut microbiota by increasing Firmicutes and Proteobacteria abundance. These changes were associated with a downregulation of orexigenic genes like cb1 and npy and an upregulation of anorexigenic genes like leptin and mc4r, leading to a significant reduction in food intake.16 In a study by Lee et al. eight weeks of supplementation with Lactobacillus rhamnosus PL60 in obese mice led to reduced leptin levels and adipose tissue mass, attributed to the probiotic’s production of conjugated linoleic acid.17 What is more, a controversial 2008 study found that direct administration of Lactobacillus supernatant into the central nervous system of rats increased leptin expression in the brain and fat tissue, reducing body mass.18 A study from Korea investigated a multi-strain formulation consisting of Bifidobacterium pseudocatenulatum, Bifidobacterium longum, and Bifidobacterium longum SPM 1207 on rats for seven weeks. The probiotic treatment was associated with reduced serum leptin levels, indicating potential anorexigenic effects.19
Further research by Falcinelli et al.16 on zebrafish fed a high-fat diet found that supplementation with Lactobacillus rhamnosus altered gut microbiota composition and appetite-modulating compounds’ secretion. It also changed the expression of anorexigenic and orexigenic genes, favouring appetite suppression.
Most studies on central appetite control pathways have been performed in rodents, but pigs offer a more comparable model to humans due to physiological similarities.20,21 Valent et al. studied the effect of a high-fat diet on brain neuropeptides in pigs, finding that Bifidobacterium breve supplementation, especially with omega-3 fatty acids, partially reversed high-fat diet effects on regulatory neuropeptides and the dopaminergic system.22
Both Forssten et al. and Bagarolli et al. (2013) found out that probiotic administration resulted in reduced feed intake.23,24

Future prospects for probiotics in appetite regulation include biotechnological modifications to deliver or produce anorexigenic compounds. Chen et al. demonstrated that genetically altered Escherichia coli secreting N-acyl-phosphatidylethanolamines (NAPEs) reduced food intake and improved metabolic health in mice on a high-fat diet.25
Human studies on probiotics and appetite modulation show varying outcomes due to the complexity of human physiology and study designs, although they have confirmed the appetite modulation mechanisms of probiotics observed in animal studies. While some studies indicate beneficial effects on appetite and metabolism, others show limited or no significant impact, highlighting the need for further research to clarify the mechanisms and efficacy of probiotics in appetite regulation and weight management.
A randomized, controlled, double-blind trial investigated the effect of Lactobacillus plantarum 299v supplementation over 6 weeks in 36 healthy, heavy smokers. Besides reducing cardiovascular disease risk factors, the probiotic treatment led to decreased leptin levels and interleukin-6 (IL-6) in 42% of the probiotic group, and reduced monocyte adhesion to endothelial cells. The authors suggested that short-chain fatty acid production by the probiotic was the mechanism behind these effects.26
A 2021 randomized trial tested whether a multi-strain probiotic formulation (Bifidobacterium, Lactobacillus, and Enterococcus) could mitigate weight gain and appetite changes due to antipsychotic therapy with olanzapine. No significant differences in appetite levels were found between the probiotic and control groups after 4, 8, and 12 weeks. However, there was a slight delay in appetite increase (19.8 vs. 25.6 days), and weight gain was delayed in the initial treatment phase (4 weeks).27 A multicenter double-blind randomized placebo-controlled trial on patients with higher body mass and abdominal visceral fat area found that supplementation with Lactobacillus gasseri SBT2055 (LG2055) for 12 weeks significantly increased adiponectin levels.28 One other bacteria species that gained lot of attention when it comes to research on anorexigenic (apetite suppressing) is Hafnia alvei. According to the research, Hafnia alvei produces a molecule called ClpB, which mimics the human anorexigenic hormone α-melanocyte-stimulating hormone (α-MSH). This mimicry can help reduce appetite and thus support weight management. A commercial product utilizing Hafnia alvei, particularly the strain HA4597, is available and represents one of the first probiotics developed through biotechnology to aid in weight loss. This innovation combines the natural benefits of probiotics with targeted biotechnological enhancements to influence satiety and body weight regulation. The research supports that the ClpB produced by Hafnia alvei HA4597 has functional anorexigenic properties, making it an effective supplement for those seeking to manage their weight through dietary means.34
On the other hand, a double-blind, randomized, placebo-controlled study on obese adolescents found that 16-week treatment with commercially available supplement VSL#3 had no significant effects on appetite-regulating hormones or other obesity-associated outcomes. This highlights the importance of specific probiotic strains and their unique mechanisms in influencing metabolic and appetite-related pathways.29
Clinical applications and implications
Based on our brief scoping review, probiotics have demonstrated anorexigenic properties, which are strain-specific. Not all probiotic strains improve appetite suppression, and their efficacy depends on genetic and metabolic characteristics. Probiotics must produce short-chain fatty acids or other essential metabolites to regulate appetite.30 They should also modulate gut microbiota and metabolome to reduce gut permeability, endotoxemia, and inflammation, crucial for appetite control. Additionally, effective probiotics induce enteroendocrine cells to produce anorexigenic metabolites and affect lipid metabolism, from absorption to adipose tissue function.16,31,32
Our review highlights that the anorexigenic effect is dose-dependent and more pronounced with long-term supplementation, typically lasting at least 12 weeks. Also, short-term treatments have shown limited efficacy.30,33
Conclusion
Appetite regulation involves a complex interplay of hormones and neuropeptides that signal hunger and fullness.
Probiotics hold great potential not only for general health but also for regulating appetite through their impact on the gut microbiome. Based on these findings, probiotics do not seem to consistently affect key appetite-related hormones in overweight and obese populations. However, the variability in study outcomes underscores the complexity of probiotic interactions with human physiology, necessitating further research to elucidate their mechanisms and potential therapeutic applications in managing weight and metabolic health. Regarding the application, we recommend long-term probiotic supplementation with a high-dose regimen, using either a multi-strain formulation as supported by various studies or a next-generation probiotic known for its anorexigenic effects, such as Hafnia alvei HA4597. Finally, continued research in this area could lead to new, effective treatments for appetite-related disorders, offering hope for those struggling with issues like obesity or undernutrition.
References:
1 Watterson, K. R., Bestow, D., Gallagher, J., Hamilton, D. L., Ashford, F. B., Meakin, P. J., & Ashford, M. L. J. (2013). Anorexigenic and orexigenic hormone modulation of mammalian target of rapamycin complex 1 activity and the regulation of hypothalamic agouti-related protein mRNA expression. NeuroSignals, 21(1–2), 28–41. https://doi.org/10.1159/000334144
2 Dixon, J. B. (2010). The effect of obesity on health outcomes. In Molecular and Cellular Endocrinology (Vol. 316, Issue 2, pp. 104–108). https://doi.org/10.1016/j.mce.2009.07.008
3 Thaler, J. P., Choi, S. J., Schwartz, M. W., & Wisse, B. E. (2010). Hypothalamic inflammation and energy homeostasis: Resolving the paradox. In Frontiers in Neuroendocrinology (Vol. 31, Issue 1, pp. 79–84). https://doi.org/10.1016/j.yfrne.2009.10.002
4 Blüher, M. (2019). Obesity: global epidemiology and pathogenesis. In Nature Reviews Endocrinology (Vol. 15, Issue 5, pp. 288–298). Nature Publishing Group. https://doi.org/10.1038/s41574-019-0176-8
5 Fantuzzi, G. (2005). Adipose tissue, adipokines, and inflammation. In Journal of Allergy and Clinical Immunology (Vol. 115, Issue 5, pp. 911–919). Mosby Inc. https://doi.org/10.1016/j.jaci.2005.02.023
6 Cummings, D. E., Purnell, J. Q., Frayo, R. S., Schmidova, K., Wisse, B. E., & Weigle, D. S. (2001). A Preprandial Rise in Plasma Ghrelin Levels Suggests a Role in Meal Initiation in Humans. Diabetes, 50(8), 1714–1719. https://doi.org/10.2337/diabetes.50.8.1714
7 Ramanjaneya, M., Chen, J., Brown, J. E., Tripathi, G., Hallschmid, M., Patel, S., Kern, W., Hillhouse, E. W., Lehnert, H., Tan, B. K., & Randeva, H. S. (2010). Identification of nesfatin-1 in human and murine adipose tissue: A novel depot-specific adipokine with increased levels in obesity. Endocrinology, 151(7), 3169–3180. https://doi.org/10.1210/en.2009-1358
8 Bagarolli, R. A., Tobar, N., Oliveira, A. G., Araújo, T. G., Carvalho, B. M., Rocha, G. Z., Vecina, J. F., Calisto, K., Guadagnini, D., Prada, P. O., Santos, A., Saad, S. T. O., & Saad, M. J. A. (2017). Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. Journal of Nutritional Biochemistry, 50, 16–25. https://doi.org/10.1016/j.jnutbio.2017.08.006
9 Carvalho, B. M., Jose, M., & Saad, A. (2013). Influence of Gut Microbiota on Subclinical Inflammation and Insulin Resistance. Mediators of Inflammation, 2013, 13. https://doi.org/10.1155/2013/986734
10 Bagarolli, R. A., Tobar, N., Oliveira, A. G., Araújo, T. G., Carvalho, B. M., Rocha, G. Z., Vecina, J. F., Calisto, K., Guadagnini, D., Prada, P. O., Santos, A., Saad, S. T. O., & Saad, M. J. A. (2017). Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. Journal of Nutritional Biochemistry, 50, 16–25. https://doi.org/10.1016/j.jnutbio.2017.08.006
11 Falcinelli, S., Picchietti, S., Rodiles, A., Cossignani, L., Merrifield, D. L., Taddei, A. R., Maradonna, F., Olivotto, I., Gioacchini, G., & Carnevali, O. (2015). Lactobacillus rhamnosus lowers zebrafish lipid content by changing gut microbiota and host transcription of genes involved in lipid metabolism. https://doi.org/10.1038/srep09336
12 Ghosh, S., van Heel, D., & Playford, R. J. (2004). Probiotics in inflammatory bowel disease: Is it all gut flora modulation? In Gut (Vol. 53, Issue 5, pp. 620–622). https://doi.org/10.1136/gut.2003.034249
13 Pražnikar, Z. J., Kenig, S., Vardjan, T., Bizjak, M. Č., & Petelin, A. (2020). Effects of kefir or milk supplementation on zonulin in overweight subjects. Journal of Dairy Science, 103(5), 3961–3970. https://doi.org/10.3168/jds.2019-17696
14 Ejtahed, H. S., & Hasani-Ranjbar, S. (2019). Neuromodulatory effect of microbiome on gut-brain axis; new target for obesity drugs. In Journal of Diabetes and Metabolic Disorders (Vol. 18, Issue 1, pp. 263–265). Springer. https://doi.org/10.1007/s40200-019-00384-4
15 Pizarroso, N. A., Fuciños, P., Gonçalves, C., Pastrana, L., & Amado, I. R. (2021). A review on the role of food-derived bioactive molecules and the microbiota–gut–brain axis in satiety regulation. In Nutrients (Vol. 13, Issue 2, pp. 1–18). MDPI AG. https://doi.org/10.3390/nu13020632
16 Falcinelli, S., Rodiles, A., Hatef, A., Picchietti, S., Cossignani, L., Merrifield, D. L., Unniappan, S., & Carnevali, O. (2017). Dietary lipid content reorganizes gut microbiota and probiotic L. rhamnosus attenuates obesity and enhances catabolic hormonal milieu in zebrafish. Scientific Reports, 7(1). https://doi.org/10.1038/s41598-017-05147-w
17 Lee, H. Y., Park, J. H., Seok, S. H., Baek, M. W., Kim, D. J., Lee, K. E., Paek, K. S., Lee, Y., & Park, J. H. (2006). Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochimica et Biophysica Acta – Molecular and Cell Biology of Lipids, 1761(7), 736–744. https://doi.org/10.1016/j.bbalip.2006.05.007
18 Sousa, R., Halper, J., Zhang, J., Lewis, S. J., & Li, W. I. O. (2008). Effect of Lactobacillus acidophilus supernatants on body weight and leptin expression in rats. BMC Complementary and Alternative Medicine, 8(1). https://doi.org/10.1186/1472-6882-8-5
19 An, H. M., Park, S. Y., Lee, D. K., Kim, J. R., Cha, M. K., Lee, S. W., Lim, H. T., Kim, K. J., & Ha, N. J. (2011). Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats. Lipids in Health and Disease, 10. https://doi.org/10.1186/1476-511X-10-116
20 Bassols, A., Costa, C., Eckersall, P. D., Osada, J., Sabrià, J., & Tibau, J. (2014). The pig as an animal model for human pathologies: A proteomics perspective. In Proteomics – Clinical Applications (Vol. 8, Issue 10, pp. 715–731). Wiley-VCH Verlag. https://doi.org/10.1002/prca.201300099
21 Koopmans, S. J., & Schuurman, T. (2015). Considerations on pig models for appetite, metabolic syndrome and obese type 2 diabetes: From food intake to metabolic disease. In European Journal of Pharmacology (Vol. 759, pp. 231–239). Elsevier B.V. https://doi.org/10.1016/j.ejphar.2015.03.044
22 Valent, D., Arroyo, L., Fàbrega, E., Font-I-Furnols, M., Rodríguez-Palmero, M., Moreno-Muñoz, J. A., Tibau, J., & Bassols, A. (2020). Effects of a high-fat-diet supplemented with probiotics and ω3-fatty acids on appetite regulatory neuropeptides and neurotransmitters in a pig model. Beneficial Microbes, 11(4), 347–359. https://doi.org/10.3920/BM2019.0197
23 Forssten, S. D., Korczyńska, M. Z., Zwijsen, R. M. L., Noordman, W. H., Madetoja, M., & Ouwehand, A. C. (2013). Changes in satiety hormone concentrations and feed intake in rats in response to lactic acid bacteria. Appetite, 71, 16–21. https://doi.org/10.1016/j.appet.2013.06.093
24 Bagarolli, R. A., Tobar, N., Oliveira, A. G., Araújo, T. G., Carvalho, B. M., Rocha, G. Z., Vecina, J. F., Calisto, K., Guadagnini, D., Prada, P. O., Santos, A., Saad, S. T. O., & Saad, M. J. A. (2017). Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. Journal of Nutritional Biochemistry, 50, 16–25. https://doi.org/10.1016/j.jnutbio.2017.08.006
25 Chen, Z., Guo, L., Zhang, Y., Walzem, R. L., Pendergast, J. S., Printz, R. L., Morris, L. C., Matafonova, E., Stien, X., Kang, L., Coulon, D., McGuinness, O. P., Niswender, K. D., & Davies, S. S. (2014). Incorporation of therapeutically modified bacteria into Gut microbiota inhibits obesity. Journal of Clinical Investigation, 124(8), 3391–3406. https://doi.org/10.1172/JCI72517
26 Naruszewicz, M., Johansson, M.-L., Zapolska-Downar, D., & Bukowska, H. (2002). Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers 1-3. In Am J Clin Nutr (Vol. 76). https://academic.oup.com/ajcn/article-abstract/76/6/1249/4689562
27 Yang, Y., Long, Y., Kang, D., Liu, C., Xiao, J., Wu, R., & Zhao, J. (2021). Effect of Bifidobacterium on olanzapine-induced body weight and appetite changes in patients with psychosis. Psychopharmacology, 238(9), 2449–2457. https://doi.org/10.1007/s00213-021-05866-z
28 Kadooka, Y., Sato, M., Imaizumi, K., Ogawa, A., Ikuyama, K., Akai, Y., Okano, M., Kagoshima, M., & Tsuchida, T. (2010). Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. European Journal of Clinical Nutrition, 64(6), 636–643. https://doi.org/10.1038/ejcn.2010.19
29 Jones, R. B., Alderete, T. L., Martin, A. A., Geary, B. A., Hwang, D. H., Palmer, S. L., & Goran, M. I. (2018). Probiotic supplementation increases obesity with no detectable effects on liver fat or gut microbiota in obese Hispanic adolescents: a 16-week, randomized, placebo-controlled trial. Pediatric Obesity, 13(11), 705–714. https://doi.org/10.1111/ijpo.12273
30 Déchelotte, P., Breton, J., Trotin-Picolo, C., Grube, B., Erlenbeck, C., Bothe, G., Fetissov, S. O., & Lambert, G. (2021). The probiotic strain h. Alvei ha4597® improves weight loss in overweight subjects under moderate hypocaloric diet: A proof-of-concept, multicenter randomized, double-blind placebo-controlled study. Nutrients, 13(6). https://doi.org/10.3390/nu13061902
31 Takemura, N., Okubo, T., & Sonoyama, K. (2010). Lactobacillus plantarum strain No. 14 reduces adipocyte size in mice fed high-fat diet. Experimental Biology and Medicine, 235(7), 849–856. https://doi.org/10.1258/ebm.2010.009377
32 Zarrati, M., Raji Lahiji, M., Salehi, E., Yazdani, B., Razmpoosh, E., Shokouhi Shoormasti, R., & Shidfar, F. (2019). Effects of Probiotic Yogurt on Serum Omentin-1, Adropin, and Nesfatin-1 Concentrations in Overweight and Obese Participants Under Low-Calorie Diet. Probiotics and Antimicrobial Proteins, 11(4), 1202–1209. https://doi.org/10.1007/s12602-018-9470-3
33 NabizadehAsl, L., Sendur, S. N., Ozer, B., Lay, I., Erbas, T., & Buyuktuncer, Z. (2022). Acute and short-term effects of Lactobacillus paracasei subsp. paracasei 431 and inulin intake on appetite control and dietary intake: A two-phases randomized, double blind, placebo-controlled study. Appetite, 169, 105855. https://doi.org/10.1016/J.APPET.2021.105855
34 Legrand R, Lucas N, Dominique M, Azhar S, Deroissart C, Le Solliec MA, Rondeaux J, Nobis S, Guérin C, Léon F, do Rego JC, Pons N, Le Chatelier E, Ehrlich SD, Lambert G, Déchelotte P, Fetissov SO. Commensal Hafnia alvei strain reduces food intake and fat mass in obese mice-a new potential probiotic for appetite and body weight management. Int J Obes (Lond). 2020 May;44(5):1041-1051. doi: 10.1038/s41366-019-0515-9
Sky Office, Toranj B
R. F. Mihanovića 9, Zagreb, Croatia
Mob.: + 385 98 13 73 344
Tel.: + 385 1 78 99 694




