Ginseng is the roots and rhizomes of Panax ginseng Mey., belonging to the perennial plants of the genus Panax and family Araliaceae. The most commonly used species are: Panax ginseng (Asian ginseng), Panax quinquefolius (American ginseng) and Panax notoginseng (Chinese notoginseng or Sanqi).
Ginseng, a revered traditional medicinal herb with a history spanning over 4000 years, has been extensively utilised for its purported health-promoting and restorative properties1,2. Commercially cultivated for the past three centuries, various preparations of ginseng, including fresh, dried, steamed, and extracted forms, are available, each containing over 50 identified ginsenosides3. These bioactive compounds are primarily responsible for the diverse pharmacological activities attributed to ginseng, encompassing its effects on the immune, endocrine, cardiovascular, and central nervous systems4.
The plant also contains other important secondary metabolites such as ginseng oils, phytosterols, carbohydrates, amino acids, peptides, vitamins, minerals, enzymes, and phenolic compounds, all contributing to its therapeutic profile1. Among these, Korean Red Ginseng is particularly notable due to the unique ginsenoside-Rg3 compound formed during its specialized steaming and drying process, which enhances its preservation and therapeutic potential4. This extensive phytochemical complexity underscores the multifaceted mechanisms through which ginseng exerts its therapeutic effects, influencing various physiological pathways, including those related to mental well-being, bone density, and reproductive health.
While the precise mechanisms underlying many of ginseng’s purported benefits remain areas of active research, its long-standing use in traditional medicine systems worldwide highlights a rich empirical foundation for its application in modern therapeutic contexts. The adaptogenic properties of ginseng are particularly relevant in the context of mental well-being, where it is believed to help the body adapt to stress and maintain homeostasis. Its potential to alleviate conditions like depression and anxiety, often linked to chronic stress, is a key area of investigation5.
Furthermore, its anti-inflammatory and immunomodulatory activities, largely attributed to ginsenosides, offer additional avenues for therapeutic intervention in conditions characterized by chronic inflammation. The comprehensive nature of its constituents allows ginseng to interact with various biological pathways, thereby offering potential therapeutic benefits across a spectrum of physiological systems. Among its many therapeutic applications, ginseng has been traditionally recognized for its ability to combat fatigue and improve overall vitality. This comprehensive action extends its utility to mental health applications, making it a promising candidate for further research into neurodegenerative and psychological disorders.
Its adaptogenic properties are particularly relevant in the context of stress-related disorders like depression. While the precise mechanisms underlying many of ginseng’s purported benefits remain areas of active research, its long-standing use in traditional medicine systems worldwide highlights a rich empirical foundation for its application in modern therapeutic contexts.
Specifically, ginseng is categorized as an adaptogen, an herbal medicinal product that enhances an organism’s resilience and adaptability to various stressors6. This classification indicates its capacity to regulate physiological processes and restore balance within the body, particularly in response to physical or psychological challenges. These adaptogenic qualities allow ginseng to modulate the body’s stress response without increasing oxygen consumption, improving overall physiological stability7 (Figure 1).
FIGURE 1 Mechanism of action of Panax ginseng7
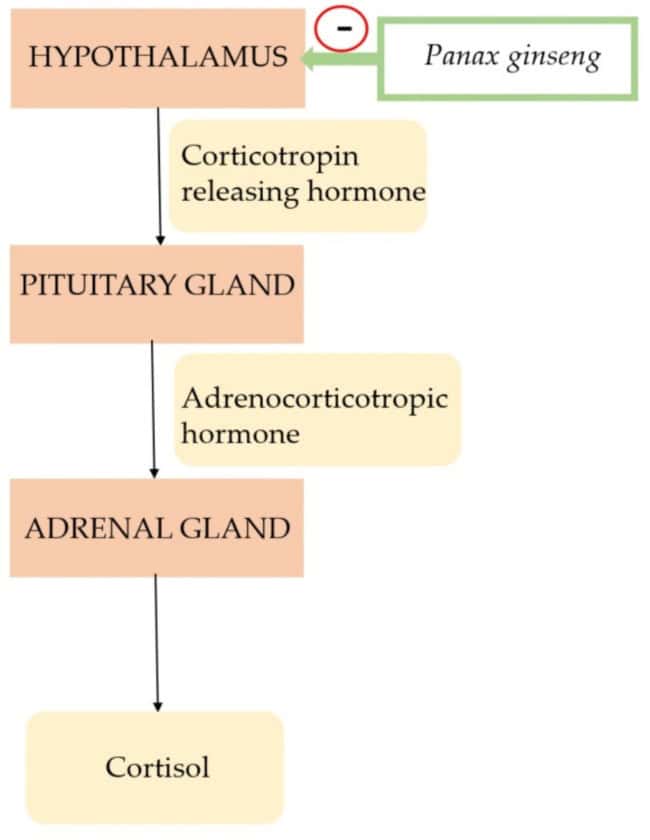
This unique characteristic differentiates adaptogens from conventional stimulants, allowing them to exert their therapeutic effects without inducing side effects such as increased heart rate or blood pressure6. This capacity to enhance stress resilience positions ginseng as a promising natural intervention for conditions exacerbated by chronic stress, including mood disorders and certain physiological dysfunctions. This ability to normalize physiological functions makes it a valuable subject for research into chronic conditions where the body’s homeostatic mechanisms are disrupted7.
Although significant progress has been made in understanding ginseng’s pharmacological actions, further rigorous clinical trials are necessary to fully clarify its therapeutic potential and establish standardised dosages for various conditions. For example, ginseng has been extensively researched for its potential in alleviating chronic fatigue syndrome, a debilitating condition with limited conventional treatments8.
Beyond its anti-fatigue properties, ginseng has also shown considerable promise in enhancing mental well-being by reducing anxiety and improving cognitive function, thereby supporting overall psychological resilience9. While current literature indicates that ginseng may improve well-being in perimenopausal women, its purity, potential side effects, and drug interactions require careful consideration10.
Ginseng and osteoporosis

Osteoporosis, a systemic disease of the bone characterized by reduced skeletal strength predisposing to a high risk of fracture, is a global public health problem. Osteoporosis is most prevalent in postmenopausal women due to the estrogen deficiency, causing an increase in bone remodeling and net loss of bone. Osteoporosis-induced bone mineral density loss can lead to increased fragility and susceptibility to fractures, accounting significantly for reduced quality of life. With estrogen’s significance to bone health, particularly that of postmenopausal women, the study of naturally occurring substances that are able to impact hormonal equilibrium or directly affect bone metabolism is of particular interest.
Estrogen deficiency is a significant contributor to osteoporosis pathogenesis in postmenopausal women, accompanied by greater bone loss and risk of fractures. The imbalance due to this hormone leads to increased bone resorption, and further aided by potential iron overload. Postmenopausal women account for the majority of osteoporosis patients, with age factors also contributing to its onset, with the latter more commonly resulting from excessive reactive oxygen species formation
By regulating the HPA axis, ginseng influences the hormonal system. One of the hormones regulated in this manner is estrogen, a sex hormone that acts as an agonist in the brain, bones, and heart. The effects of estrogen reduce the risk of developing CVD, stroke, and osteoporosis. Ginseng has also been shown to upregulate the estrogen receptor in a range of cell types in vitro, indicating that it could increase the tissue effects of this hormone. This phenomenon is observed in bone tissue.
In postmenopausal women, where there is a sudden decline in estrogen levels, the probability of osteoporosis is proportionally increased. The bone tissue of patients with osteoporosis can fracture easily owing to a reduced bone density. This is related to the amount of estrogen supplied to osteoclasts. Estrogen inversely regulates the expression of interleukin (IL)-6, a proinflammatory cytokine. Chronic increases in the level of IL-6 result in osteoporosis and similar increases can also be observed in patients with depression or sleep disorders, or those experiencing stress because of bad eating habits.
Ginseng promotes osteogenesis in the bone marrow stromal cell. In addition, by inhibiting receptor activator of nuclear factor kappa-B ligand, nuclear factor kappa-light-chain-enhancer of activated B cells, c-Jun N-terminal kinases, c-Fos, nuclear factor of activated T cells, cytoplasmic 1 (NFATc1), and the proinflammatory cytokines, tumor necrosis factor alpha (TNF-α) and IL-6, and by playing a role in osteoclast differentiation and bone resorption, it helps to prevent osteoporosis. However, further research is required to elucidate the exact mechanisms underlying these effects of ginseng5.
Phytoestrogens, naturally occurring compounds with estrogen-like activity, are promising avenue for therapeutic intervention in post-menopausal osteoporosis. These compounds, found in various plants, can bind to estrogen receptors, potentially mitigating the adverse effects of estrogen deficiency on bone density.
In this context, ginseng, possessing phytoestrogenic properties, has garnered attention as a potential natural alternative to traditional hormone therapy, warranting further investigation into its mechanisms of action and clinical utility in mitigating bone loss. Therefore, exploring ginseng’s multifaceted effects on bone metabolism, beyond its phytoestrogenic aspects, is crucial for understanding its full therapeutic potential in managing postmenopausal osteoporosis. Moreover, the vascular system plays an integral role in bone maintenance, with common risk factors for cardiovascular disease often overlapping with those for osteoporosis, despite sex-specific predispositions11.
Consequently, understanding the influence of ginseng on both bone remodelling and cardiovascular health in the context of estrogen deficiency is paramount. This emphasizes the growing interest in botanical compounds, like ginseng, which may offer a safer, yet effective, approach to managing menopausal symptoms and mitigating osteoporosis risk. This underscores the importance of investigating ginseng’s pleiotropic effects, not solely on bone density, but also on broader aspects of physiological well-being affected by hormonal fluctuations.

Furthermore, the structural similarities between phytoestrogens and 17-β-estradiol, the primary female sex hormone, suggest a plausible mechanism for ginseng’s potential to alleviate menopausal symptoms and reduce the risk of osteoporosis12. This interaction is primarily mediated through their binding to estrogen receptors alpha and beta, which are widely distributed throughout various tissues, including bone, brain, and the urogenital tract, allowing for diverse physiological responses.
This broad distribution of estrogen receptors enables phytoestrogens to exert pleiotropic effects, influencing not only bone homeostasis but also neuroprotection, cardiovascular function, and other systemic processes modulated by endogenous estrogens14,15. Widespread receptor engagement underscores the potential for ginseng-derived phytoestrogens to exert systemic benefits that extend beyond bone health, influencing mental well-being and sexual health by mimicking estrogenic actions in various target tissues. This mimicry can be particularly beneficial for neuroprotective effects, safeguarding the brain against conditions like dementia and complications from traumatic injury, while also impacting cardiovascular health16.
The influence of ginseng’s active compounds on estrogen receptor modulation thus provides a compelling mechanistic basis for its investigation as a therapeutic agent for menopausal symptoms, encompassing bone health, cognitive function, and cardiovascular integrity. Specifically, certain natural products, including isoflavones, prenylflavones, coumestans, stilbenes, and lignans, exhibit endocrine perturbations, particularly on the estrogenic hormonal pathway, due to their structural analogies to estradiol. Among these, isoflavones, commonly found in soy products, have been extensively studied for their phytoestrogenic properties and their potential to mitigate estrogen-deficiency related conditions17,18. However, the specific composition and concentration of these phytoestrogens in various ginseng species, and their subsequent bioavailability and bioactivity, remain crucial considerations for their therapeutic application19.
Ginseng and sex-specific health effects
The differential binding affinities of various phytoestrogens to estrogen receptor alpha and beta, along with their tissue-specific expression patterns, contribute to the diverse physiological outcomes observed. This variability necessitates a comprehensive understanding of how different ginseng preparations, rich in distinct phytoestrogen profiles, selectively modulate estrogen receptor pathways to achieve targeted health benefits without inducing undesirable side effects.
This understanding is paramount for optimizing ginseng formulations for specific therapeutic indications, ranging from osteoporosis management to cognitive enhancement and improvement in sexual health, thereby advancing personalized medicine approaches. The intricate interplay between phytoestrogens and estrogen receptors dictates the efficacy and safety of ginseng in ameliorating age-related declines in health. This nuanced understanding of receptor kinetics and ligand specificity is essential for developing standardized ginseng extracts that reliably deliver desired clinical outcomes for both women and men20.
Comprehensive mechanistic understanding is crucial for bridging the gap between traditional medicinal uses of ginseng and its evidence-based integration into modern clinical practice, especially given the recently acknowledged importance of estrogens in male physiology, particularly in bone metabolism and reproduction. This includes the recognition that circulating estrogen levels in men can be comparable to those in women, underscoring their vital, multifaceted roles beyond traditionally female-centric functions21. This broad physiological relevance of estrogens, coupled with the ubiquitous presence of estrogen receptors, solidifies the rationale for exploring ginseng’s modulatory effects on these pathways in both sexes, thereby expanding its therapeutic potential for a broader range of age-related conditions.
However, despite increasing interest in natural compounds, there remains a notable research gap concerning sex-specific pharmacological responses, as women often experience more frequent and severe adverse drug reactions compared to men, highlighting the need for tailored therapeutic approaches. This disparity underscores the critical need for gender-specific pharmacodynamic and pharmacokinetic studies to optimize dosing and minimize risks associated with ginseng supplementation in both sexes. Such investigations are particularly pertinent given the established sex-dependent differences in cardiovascular disease progression, where physiological concentrations of estradiol are known to exert cardioprotective effects predominantly in pre-menopausal women through estrogen receptor-mediated modulation of the immune system22.
Furthermore, estrogen metabolism and receptor signaling pathways are increasingly recognized as critical modifiers in the development of conditions like pulmonary arterial hypertension, revealing a complex interplay that warrants sex-specific therapeutic consideration. This highlights the importance of elucidating how ginseng’s phytoestrogens might influence these estrogenic pathways in a sex-dependent manner. The differential effects of estrogens and androgens on the renin-angiotensin system further underscore the necessity of sex-specific research to fully understand ginseng’s potential in mitigating cardiovascular disease risk in both men and women. This underscores the necessity for clinical trials to report sex-specific outcomes, addressing the current inadequacy where less than half of such trials provide this crucial data.
Releasing the precision advantage of ginseng in ageing
The future of ginseng research lies in scientifically exploring its potential in age-related health decline with a keen focus on sex-specific mechanisms of action. There are indications to suggest that men and women respond differently to interventions due to differences in hormonal profiles, genetics, and epigenetics—factors that play an important role in cardiovascular, metabolic, and neurological health.
This perspective is particularly pertinent in those conditions where gender differences are best documented, for instance, cardiovascular disease and hypertension. For instance, although the cardioprotective effect of estrogen is well documented, the molecular basis of differential disease incidence and outcome between women and men is not clear. Moreover, women are more likely to experience age-accelerated blood pressure elevation, particularly after menopause. Such discrepancies require individualized therapy with consideration of sex as well as hormonal status.
By exploring ginseng’s effect along such physiological axes, researchers and developers can inform precision nutrition and personalized medicine approaches. More understanding of the multi-system effects of ginseng will enable the development of sex- and hormonal stage-specific interventions for:
- Optimizing efficacy by sex and hormonal stage
- Reducing the risk of side effects
- Bridging gaps created by historical gender bias in clinical studies.
For the nutraceutical and functional health segment, this is enormous opportunity given: leveraging ginseng as a platform ingredient for tailored solutions in healthy aging. This type of innovation is consistent with the principles of pharmacogenomics and growing consumer interest in evidence-based, personalized health products.
Ultimately, a sex-based study model will not only deepen our insight into the complex therapeutic profile of ginseng but drive equitable, effective, and market-appropriate solutions – bringing personalized medicine ever closer.
References
- Metwaly, A.M. et al. (2019) “Black Ginseng and Its Saponins: Preparation, Phytochemistry and Pharmacological Effects,” Molecules. Multidisciplinary Digital Publishing Institute, p. 1856. doi:10.3390/molecules24101856.
- Hou, W. et al. (2020) “Effects of Ginseng on Neurological Disorders,” Frontiers in Cellular Neuroscience. Frontiers Media. doi:10.3389/fncel.2020.00055.
- Davis, M.P. and Behm, B. (2019) “Ginseng: A Qualitative Review of Benefits for Palliative Clinicians,” American Journal of Hospice and Palliative Medicine®. SAGE Publishing, p. 630. doi:10.1177/1049909118822704.
- Song, J. et al. (2024) “The beneficial potential of ginseng for menopause,” Journal of Ginseng Research. Elsevier BV, p. 449. doi:10.1016/j.jgr.2024.05.008.
- Lee, S. and Rhee, D. (2017) “Effects of ginseng on stress-related depression, anxiety, and the hypothalamic–pituitary–adrenal axis,” Journal of Ginseng Research. Elsevier BV, p. 589. doi:10.1016/j.jgr.2017.01.010.
- Panossian, A. and Brendler, T. (2020) “The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections,” Pharmaceuticals. Multidisciplinary Digital Publishing Institute, p. 236. doi:10.3390/ph13090236
- Provino, R. (2010) “The Role of Adaptogens in Stress Management,” Australian journal of medical herbalism, 22(2), p. 41. Available at: http://www.natural-knowhow.com/home/wp-content/uploads/2015/06/Medicinal-herbs-with-Adaptogen-stress-relief-action-article.pdf (Accessed: July 2025).
- Yang, J. et al. (2022) “Ginseng for the Treatment of Chronic Fatigue Syndrome: A Systematic Review of Clinical Studies,” Global Advances in Health and Medicine. SAGE Publishing. doi:10.1177/2164957×221079790.
- Ghorbani, Z. et al. (2019) “The effect of ginseng on sexual dysfunction in menopausal women: A double-blind, randomized, controlled trial,” Complementary Therapies in Medicine, 45, p. 57. doi:10.1016/j.ctim.2019.05.015.
- Tesch, B.J. (2003) “Herbs commonly used by women: An evidence-based review,” American Journal of Obstetrics and Gynecology. Elsevier BV. doi:10.1067/mob.2003.402.
- Hendriks, M. and Ramasamy, S.K. (2020) “Blood Vessels and Vascular Niches in Bone Development and Physiological Remodeling,” Frontiers in Cell and Developmental Biology. Frontiers Media. doi:10.3389/fcell.2020.602278.
- Harlap, S. (1992) “The benefits and risks of hormone replacement therapy: An epidemiologic overview,” American Journal of Obstetrics and Gynecology. Elsevier BV, p. 1986. doi:10.1016/0002-9378(92)91399-u.
- Gorzkiewicz, J., Bartosz, G. and Sadowska‐Bartosz, I. (2021) “The Potential Effects of Phytoestrogens: The Role in Neuroprotection,” Molecules. Multidisciplinary Digital Publishing Institute, p. 2954. doi:10.3390/molecules26102954.
- Wend, K., Wend, P. and Krum, S.A. (2012) “Tissue-Specific Effects of Loss of Estrogen during Menopause and Aging,” Frontiers in Endocrinology, 3. doi:10.3389/fendo.2012.00019.
- Sochocka, M. et al. (2023) “Cognitive Decline in Early and Premature Menopause,” International Journal of Molecular Sciences. Multidisciplinary Digital Publishing Institute, p. 6566. doi:10.3390/ijms24076566.
- Farkas, S. et al. (2022) “Estradiol and Estrogen-like Alternative Therapies in Use: The Importance of the Selective and Non-Classical Actions,” Biomedicines. Multidisciplinary Digital Publishing Institute, p. 861. doi:10.3390/biomedicines10040861
- Bernatonienė, J., Kazlauskaitė, J.A. and Kopustinskienė, D.M. (2021) “Pleiotropic Effects of Isoflavones in Inflammation and Chronic Degenerative Diseases,” International Journal of Molecular Sciences. Multidisciplinary Digital Publishing Institute, p. 5656. doi:10.3390/ijms22115656.
- Domínguez‐López, I. et al. (2020) “Effects of Dietary Phytoestrogens on Hormones throughout a Human Lifespan: A Review,” Nutrients. Multidisciplinary Digital Publishing Institute, p. 2456. doi:10.3390/nu12082456.
- Chavda, V.P. et al. (2024) “Phytoestrogens: Chemistry, potential health benefits, and their medicinal importance,” Phytotherapy Research, 38(6), p. 3060. doi:10.1002/ptr.8196.
- Powers, C.N. and Setzer, W.N. (2015) “A molecular docking study of phytochemical estrogen mimics from dietary herbal supplements,” In Silico Pharmacology, 3(1). doi:10.1186/s40203-015-0008-z.
- Alemany, M. (2022) “The Roles of Androgens in Humans: Biology, Metabolic Regulation and Health,” International Journal of Molecular Sciences. Multidisciplinary Digital Publishing Institute, p. 11952. doi:10.3390/ijms231911952.
- Rosenzweig, R. et al. (2021) “Estrogenic bias in T-Lymphocyte biology: Implications for cardiovascular disease,” Pharmacological Research. Elsevier BV, p. 105606. doi:10.1016/j.phrs.2021.105606.




