Introduction
Glucans are among the most abundant organic polymers on Earth. Due to the bulkiness and rigidity of glucose, the basic monomer from which they are composed, glucans are also structurally rigid molecules (1). Their unique properties primarily arise from the specific glycosidic bonds linking individual glucose units. In nature, glucans play an important role in numerous physiological processes (2).
Glucans with α-glycosidic bonds are primarily involved in intracellular energy storage, as is the case with starch (α-1,4-glucan). In contrast, β-glycosidically linked glucans typically participate in the formation of structural components of cell walls, such as in cellulose (β-1,4-glucan). Both isomers of glycosidic linkage, α and β, are also found in microbial exopolysaccharides, where they play a key role in cellular protection, adherence to solid surfaces, and intercellular communication (2,3). β-glucan stands out as a particularly promising bioactive compound due to its beneficial properties, which are comparable to and in some cases superior to those of other polysaccharides (4).
Βeta-glucan

β-glucan is considered a bioactive food component due to its diverse biological activities. It is naturally found in various sources such as bacteria, fungi, algae, cereals (barley and oats), and yeast. Additional sources include marine algae and various medicinal mushrooms (e.g., Lentinula edodes – Shiitake, Ganoderma lucidum – Lingzhi, Inonotus obliquus – Chaga, Grifola frondosa – Maitake), which have been the focus of research due to their potential immunomodulatory and antitumor properties.
Structure and bioactivity of β-glucans
Although the basic structural units of β-glucans from different sources exhibit a high degree of similarity, numerous studies indicate that their biological activities can vary significantly. This variability arises from differences in polymer chain length, type and position of glycosidic linkages, degree and pattern of branching, and conformational (three-dimensional) structure, despite the presence of common β-glycosidic bonds between glucose units. These structural features play a crucial role in determining the biological activity and functional properties of individual β-glucans. In aqueous solutions, polysaccharides can form complex supramolecular structures due to intra- and intermolecular interactions, such as hydrogen bonding, including random coils, single, double, and triple helices, as well as aggregates like short rods and filamentous structures.
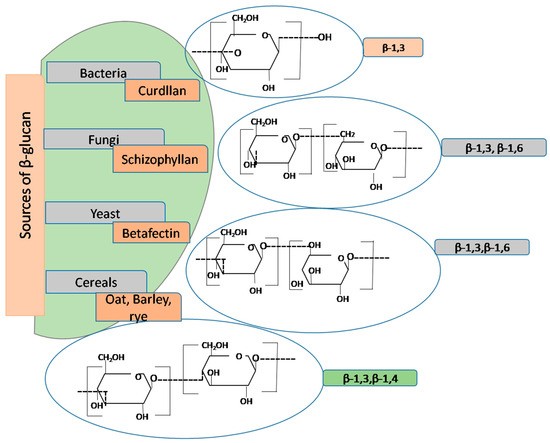
β-glucans are polymers composed of glucose subunits linked by glycosidic bonds, often branched at the sixth position of the backbone. They primarily consist of a linear core of β-1,3-d-glucose units, with a variable degree of branching involving side β-1,4 or β-1,6 glycosidic linkages, depending on the biological source. Yeasts and fungi contain β-1,6 branches, while cereals contain β-1,4 side chains. Depending on their origin, there are variations in the structural complexity of β-glucan molecules, their impact on biological activity, pharmacological potential, and physicochemical properties such as solubility, viscosity, gelling capacity, and molecular weight. The degree of branching of β-glucans from different sources is shown in Figure 1.
FIGURE 1 Schematic representation of the structure and degree of branching of β-glucans from different sources (11)
β-glucans exhibit diverse mechanisms of biological activity that are directly related to the specific features of their conformation, i.e., to well-defined structure–function relationships. Among the most well-known biologically active fungal polysaccharides are schizophyllan, lentinan, and scleroglucan, which, under most experimental conditions, form a stable triple-helix conformation.
Immune system
Historical records testify to the millennia-long use of mushrooms for medicinal purposes. Ancient Indian texts dating back approximately 5000 years, Japanese legends about Lentinula edodes, as well as the practices of African shamans and Native Americans, point to an early intuitive recognition of their beneficial effects.
Immunomodulators are substances capable of modulating the immune response – either by enhancing or suppressing it. They include a broad range of natural, synthetic, and recombinant molecules, such as curcumin, resveratrol, flavonoids, and plant extracts. However, comprehensive scientific research is lacking for the majority of natural immunomodulators. Comparative studies have shown that β-glucans consistently exhibit strong biological effects.
β-1,3-glucans (glucans) are highly conserved pathogen-associated molecular patterns (PAMPs) that the immune system recognizes as foreign structures. The first reports of their immunomodulatory activity date back to the 1940s, when a polysaccharide extracted from Serratia marcescens was found to possess antitumor properties. Subsequent studies confirmed the presence of glucose residues within it. For safety reasons, research later shifted to glucans derived from non-toxic sources such as mushrooms, yeasts, and plants. Evolutionarily, the ability to recognize glucans is present across all multicellular organisms, from invertebrates to humans.
Glucans are considered potent activators of cellular immunity, with macrophages being the most important biological targets. Early studies demonstrated that glucan provided protection against infections caused by Leishmania major, L. donovani, Candida albicans, Toxoplasma gondii, Streptococcus suis, Plasmodium berghei, Staphylococcus aureus, Escherichia coli, Mesocestoides corti, Trypanosoma cruzi, Eimeria vermiformis i Bacillus anthracis (19).
Antitumor and immunomodulatory effects
Cancer is a complex and heterogeneous group of diseases characterized by uncontrolled cell proliferation and represents one of the greatest challenges in modern medicine. It is the second leading cause of death worldwide, accounting for approximately one in six deaths, with nearly 19.3 million new diagnoses and about 10 million deaths annually. Standard therapies – surgery, chemotherapy, and radiotherapy – are still associated with significant side effects and limited efficacy.
β-glucan demonstrates pronounced anticarcinogenic potential through immunomodulatory mechanisms that prevent oncogenesis and inhibit the metastatic development of tumors. Its action is based on a multifaceted approach: direct inhibition of tumor growth, stimulation of the immune response, and intrinsic antitumor properties. The effectiveness of β-glucan depends on its source, molecular structure, branching pattern, and chemical modifications.
The immune response encompasses innate and adaptive mechanisms involved in the recognition and elimination of pathogens and the maintenance of organismal homeostasis. High-molecular-weight β-glucans, particularly those from fungi and yeasts with a high degree of branching and (1→3)- and (1→6)-β-glycosidic linkages, exhibit potent immunomodulatory activity primarily by acting on components of the innate immune system. Due to their repetitive structure, β-glucans bind to specific receptors on immune cells, including dectin-1 (mainly insoluble β-glucans), CR3, and TLR2 (mainly soluble β-glucans), thereby activating a signaling cascade that results in enhanced phagocytosis, antigen presentation, production of reactive oxygen species (ROS), and secretion of cytokines and chemokines. These secreted cytokines further stimulate the adaptive immune response by activating B and T lymphocytes, thus promoting both humoral and cell-mediated immunity.
β-glucans can modulate the tumor microenvironment (TME) by transforming immunosuppressive cells into immunostimulatory ones, thereby enhancing the antitumor immune response. Clinical studies have shown that combining glucans (e.g., PGG, a derivative of YBG) with monoclonal antibodies improves remission outcomes and reduces tumor burden in patients with small-cell lung cancer. Furthermore, polysaccharides from Ganoderma lucidum have been shown to induce dendritic cell maturation and modulate the cytokine profile in lung cancer patients after 12 weeks of therapy. To increase the anticarcinogenic efficacy of β-glucans, it is crucial to ensure their precise distribution within the body and effective interaction with the immune system, underscoring the importance of understanding β-glucan pharmacokinetics and trafficking mechanisms.
Although oral administration is the most common mode of intake, β-glucans can also be administered intraperitoneally (IP) or intravenously (IV), though these routes are less common.
After oral administration, β-glucans reach the proximal part of the small intestine, where they are phagocytosed by intestinal epithelial cells or M cells (microfold pinocytic cells). They are then transported to immune cells in Peyer’s patches, where they are taken up by gastrointestinal macrophages. These macrophages then migrate via the bloodstream to lymph nodes, where β-glucan activates dendritic cells. Dendritic cells capture and phagocytose damaged tumor cells within the tumor microenvironment, which stimulates the activation and differentiation of antigen-specific CD4+ and CD8+ T lymphocytes. Degraded β-glucan fragments further activate neutrophils by binding to complement receptor 3 (CR3), while simultaneously modulating myeloid progenitor cells in the bone marrow. These processes trigger CD3/CR3-dependent cellular cytotoxicity (CR3-DCC) directed against opsonized tumor cells coated with iC3b.
Orally administered β-glucan additionally induces a respiratory burst, increases phagocytosis rates, and enhances the secretion of key cytokines, including interleukin-1 (IL-1), IL-6, and TNF-α, within macrophages. It also regulates the acute phase of the humoral immune response and increases the activity of lysozyme and ceruloplasmin in animal models.
All these mechanisms collectively confirm the multidimensional potential of β-glucans in oncological immunotherapy and immunomodulation.
Role of β-glucan in the gut microbiota
The gut microbiota, often referred to as the “second brain,” is crucial for maintaining health. Its ability to break down β-glucan, a type of dietary fiber (DF), contributes to gut homeostasis. An imbalance in the microbiota can increase intestinal permeability, cause inflammation, and contribute to the development of colorectal cancer. The microbiota comprises more than 100 trillion microorganisms involved in various processes, including vitamin biosynthesis, angiogenesis, and metabolic regulation.
Indigestible polysaccharides like β-glucan promote the growth of beneficial bacteria (e.g., Lactobacillus and Bifidobacterium) and the production of short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate, which have a positive effect on gut health. Fermentation also creates beneficial derivatives such as indoles and secondary bile acids. The breakdown of β-(1,3)-glucan is facilitated by microbiota enzymes like β-(1,3)-glucosidase and β-(1,3)-glucanase, while β-(1,6)-linked structures are broken down by β-(1,6)-glucanase. These enzymes ensure nutrient availability and promote immune balance.
Studies in animals and humans show that β-glucan intake improves body mass, antioxidant activity, and reduces inflammation. A clinical trial involving 52 adult participants demonstrated that daily intake of 0.75 g of barley β-glucan for 30 days is safe and significantly increases the number of bifidobacteria.
Stability of β-glucan in the GIT
β-glucan is a highly polar macromolecule with a large molecular weight, which prevents its transmembrane transfer by passive diffusion. After oral administration, β-glucan passes through the gastrointestinal tract without significant degradation – it is resistant to the hydrolytic action of enzymes present in saliva, gastric juice, and small intestine enzymes. However, in the colon, β-glucan undergoes fermentation by the gut microbiota, where it is broken down into short-chain fatty acids (SCFAs), including acetic, propionic, and butyric acids, which are key metabolites of microbial fermentation.
Due to its molecular architecture, which lacks substrate sites for the action of human digestive enzymes, β-glucan demonstrates high stability in the gastrointestinal environment, highlighting its potential as a candidate for targeted colonic delivery of bioactive compounds. Furthermore, in vivo studies suggest that orally administered β-glucan is biologically active and comparable to its parenteral forms.
Experimental data show that, three days after the oral administration of labeled β-glucan (in soluble or particulate form), the signal is detected in the macrophages of the spleen, and the size of the ingested β-glucan corresponds to the initial molecular form. These findings suggest that phagocytosed β-glucan undergoes slow intracellular degradation, gradually releasing soluble, biologically active fragments that may act on surrounding cells. Complete degradation of β-glucan by macrophages is estimated to take approximately thirteen days, with macrophages retaining β-glucan in an undegraded state for the first four days, followed by gradual fragmentation into less soluble components.
Effect of β-glucan on health conditions
Regulation of metabolic syndrome – Metabolic syndrome encompasses a group of interrelated metabolic disorders, including abdominal obesity, atherosclerosis, low HDL cholesterol levels, glucose intolerance, elevated blood pressure, and insulin resistance. Cereal β-glucans (CBG), particularly those from oats and barley, have demonstrated effectiveness as dietary fibers in improving key metabolic parameters such as dyslipidemia and insulin resistance, along with additional antioxidant effects through the elimination of reactive oxygen species (ROS) and modulation of the gut microbiota.
The therapeutic effects of CBG are attributed to their unique structure, which includes (1→3)- and (1→4)-β-D-glucosidic bonds, making them resistant to degradation and absorption in the small intestine. By increasing the viscosity of intestinal content, CBG binds to bile acids, reduces cholesterol and triglyceride absorption, and promotes their elimination. OBG (β-glucans from oats) slow gastric emptying in healthy individuals, leading to a reduced glycemic response and decreased postprandial insulin secretion. In experiments on mice with obesity-induced diets, it has been shown that BBG (barley β-glucans) stimulate the secretion of gut hormones through fermentation products like short-chain fatty acids (SCFAs), suppress appetite, and improve insulin sensitivity.
Mechanism of hypoglycemic effect – Diabetes mellitus, a chronic metabolic disease characterized by elevated blood glucose levels, is a significant risk factor for the development of cardiovascular diseases such as hypertension, coronary artery disease, peripheral vascular disease, and atherosclerosis. According to the WHO, the prevalence of type 2 diabetes is rising globally at an alarming rate.
The etiology of the disease involves β-cell dysfunction, insulin resistance, mitochondrial dysfunction, and apoptosis. In this context, β-glucan stands out as a bioactive compound that can modulate postprandial glycemia and insulin activity. β-glucans from cereals, particularly oats and barley, have been extensively studied for their ability to lower blood glucose and cholesterol levels. Barley, a grain with a low glycemic index, shows particularly favorable effects – the addition of 4 g of β-glucan to chapatti reduced the glycemic index from 54 to 30. These effects are the result of the properties of β-glucan as a dietary fiber (DF), which slows gastric emptying and delays glucose absorption. As a dietary fiber, β-glucan is resistant to digestion in the small intestine and reaches the colon, where it undergoes microbial fermentation, producing short-chain fatty acids (SCFAs). SCFAs contribute to the maintenance of intestinal pH homeostasis and mediate the regulation of gastrointestinal hormone secretion, thereby participating in neuroendocrine signaling of satiety.
According to a study, β-glucan shows significant antidiabetic potential, as it was observed to lower glucose, cholesterol, and triglyceride levels in an obese rat model. Furthermore, oat β-glucans demonstrated the ability to inhibit intestinal disaccharidase activity, reducing the glycemic response in a dose-dependent manner, as confirmed in both in vivo and in vitro conditions. These effects are attributed to the viscous structure of the β-glucan gel, whose viscosity is inversely proportional to the postprandial rise in blood glucose.
Mechanism and effect of cholesterol lowering – Dyslipidemia, a condition characterized by elevated lipid levels in the blood, is a significant risk factor for the development of cardiovascular diseases, including myocardial infarction, stroke, and atherosclerosis. Given its high prevalence – with 11.7% of adults in the US having total cholesterol levels greater than 240 mg/dL – it is essential to explore effective therapeutic strategies.
β-glucan has emerged as a promising hypolipidemic agent that works through multiple mechanisms: it lowers cholesterol, increases the production of short-chain fatty acids (SCFAs), promotes the growth of beneficial microbiota, and contributes to colon health. The primary mechanism of β-glucan involves binding bile acids, thereby promoting their elimination through feces and consequently reducing cholesterol and bile acids in the plasma. Increased excretion of bile acids stimulates their re-synthesis in the liver, which activates cholesterol catabolism and reduces lipid absorption, further contributing to the reduction of plasma cholesterol levels. In addition, fermentation of β-glucan in the colon produces acetate and butyrate, which inhibit cholesterol synthesis. Moreover, the viscosity of the β-glucan gel slows the absorption of fats and cholesterol in the digestive system.
Clinical trials have shown that a daily intake of 3 g of β-glucan significantly reduces LDL cholesterol (~10–15%), without affecting HDL. Additionally, the high viscosity of β-glucan plays an important role in modulating the absorption of cholesterol, fats, and other biomolecules in the gastrointestinal tract, by increasing content density and slowing digestion. Similar effects are confirmed by a meta-analysis of 14 studies on barley β-glucan. Based on the available evidence, the FDA has approved health claims regarding the effect of β-glucans (oats, psyllium) on lowering blood cholesterol.
Application of β-glucan
In recent years, β-glucan has gained significant attention for its application in the food, medical, and cosmetic industries. In food science, it is used as a functional ingredient that improves the texture, stability, and nutritional value of products. Due to its immunomodulatory properties, β-glucan stands out in medicine, offering potential therapeutic benefits for diseases such as cardiovascular conditions and diabetes. In cosmetics, it is used for soothing and hydrating the skin, providing natural and effective care. The versatility of β-glucan makes it a key resource for innovation across various industrial sectors.
Application in the food and beverage industry – β-glucan is a high-molecular polysaccharide known for its gelling, thickening, and emulsifying properties, which contribute to the texture, stability, and nutritional profile of food and beverages. In culinary applications, it is used as a fat replacer and a source of soluble fiber, particularly in low-calorie formulations. Its application spans a range of food products, including ice creams, sauces, meat emulsions, fermented dairy products, and bread. However, the technological application of β-glucan is limited by its high viscosity, complex handling, and unfavorable rheological properties in industrial processes.
Recent research highlights its potential as a functional ingredient in bread enriched with resistant starch and folates, as well as in dairy products with reduced caloric value. Special attention is given to its prebiotic effects and potential for encapsulating bioactive substances. The concentration of β-glucan in food products varies significantly (0.3–20%), depending on the matrix and processing technology, with the addition affecting rheological and sensory properties, including hardness, cohesion, and elasticity.
Although β-glucan has been shown to effectively improve the nutritional quality, texture, and stability of products, optimal formulations and technological strategies for its incorporation still require further research. It is particularly important to investigate its behavior at different concentrations, interactions with proteins and other hydrocolloids, and its impact on the gut microbiota. Additionally, it is crucial to optimize sensory-acceptable formulations to ensure consumer acceptance and long-term health benefits.
Application of β-glucan in the cosmetic industry – β-glucan, a polysaccharide naturally found in fungi, cereals, and microorganisms, has gained widespread use in the cosmetic industry due to its numerous bioactive properties. Scientific research shows that β-glucan possesses soothing, moisturizing, and anti-irritant properties, making it highly beneficial for skin care. Its ability to regenerate the skin and retain moisture makes it effective in improving skin and mucosal hydration, contributing to revitalization and the maintenance of optimal skin condition.
Additionally, β-glucan is used in cosmetic formulations to improve skin health, including treatments for reducing signs of aging, such as fine lines and wrinkles caused by oxidative damage. Research suggests that β-glucan can help stimulate collagen production, resulting in reduced fine lines and alleviation of conditions such as eczema. Furthermore, β-glucan has been shown to be effective in wound healing, and studies also suggest its potential use in wound dressings in combination with chitosan. Due to its moisturizing properties, β-glucan from various sources, such as yeast and oats (e.g., “Avenacare”), is utilized in cosmetics and personal care products. Its efficacy in stimulating keratinocyte growth and protecting the skin from detergent-induced damage further demonstrates its versatility.
Application of β-glucan in the medical industry – β-glucans have become key compounds in the medical industry, with significant potential for the therapy and treatment of various diseases. Clinical trials from the 1980s marked the beginning of the use of fungal β-glucans as adjunctive therapy in cancer treatment, opening up possibilities for innovative approaches in medical treatment. β-glucans, such as lentinan, D-fraction, and schizophyllan, have shown strong anticancer effects by inhibiting the proliferation of cancer cells, inducing cytotoxicity, and promoting apoptosis through complex molecular pathways. While cancer remains one of the leading causes of death worldwide, β-glucans offer an alternative to conventional therapies like chemotherapy and radiation, with increasing emphasis on their ability to reduce metastasis and recurrence.
In addition to their direct application in cancer treatment, β-glucans possess immunomodulatory properties, which include the activation of NK cells, T cells, and macrophages, enhancing the immune response to tumor cells. Furthermore, research has shown that β-glucans can improve phagocytosis and the release of IL-2, helping to control growth. β-glucans are also promising in the treatment of infections, including COVID-19, where they have shown the ability to reduce disease symptoms and improve the immune response to SARS-CoV-2.
The effects of β-glucans in the medical industry are not limited to oncology and infections; they also possess the ability to accelerate wound healing through stimulation of macrophage infiltration, collagen synthesis, and skin reepithelialization. Clinical trials have shown that β-glucans can speed up ulcer healing, with clinical results showing a significant advantage over the control group. Additionally, β-glucans have been associated with lowering LDL cholesterol levels, and their role in regulating bile acid metabolism may play an important role in the prevention of cardiovascular diseases.
Conclusion
β-glucan is a natural polysaccharide with pronounced bioactive properties—immunomodulatory, antitumor, prebiotic, and metabolic protective. Its resistance to digestion enables fermentation in the large intestine, producing beneficial metabolites that support the gut microbiota, immune balance, and epithelial barrier. It promotes the growth of beneficial bacteria such as Lactobacillus and Bifidobacterium.
It also plays a crucial role in the regulation of metabolic syndrome through its hypoglycemic (reduced glucose absorption) and hypolipidemic effects (lowering LDL cholesterol and triglycerides), particularly with a daily intake of ≥3 g of β-glucan. Additionally, its ability to modulate the tumor microenvironment makes it a promising candidate for cancer immunotherapy.
Due to these properties, β-glucan finds widespread use in the food, medical, and cosmetic industries—as a functional fiber, immunostimulant, wound healing agent, and skin regeneration promoter. However, further research is needed to optimize its application and formulations in various fields.
References:
- MJ Gidley i K. Nishinari (2009). Chemistry, biochemistry and biology of 1-3 beta glucans and related polysaccharides. Elsevier,47–118.
- AL Rae , PJ Harris , A. Bačić i AE Clarke (1985). Planta. 166 , 128 —133.
- HR Huwyler , G. Franz i H. Meier (1978). Plant Sci. Lett. , 12 , 55 —62.
- H Zhang, J Zhang, Y Liu, C Tang (2023). Recent advances in the preparation, structure, and biological activities of β-glucan from ganoderma species: a review Foods., 12 (15) p. 2975.
- X Meng, H Liang, L Luo (2016). Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. , 424, 30–41.
- N Ohno, M Uchiyama, A Tsuzuki, K Tokunaka, NN Miura, Y Adachi, MW Aizawa, H Tamura, S Tanaka, T Yadomae (1999). Solubilization of yeast cell-wall β-(1→3)-D-glucan by sodium hypochlorite oxidation and dimethyl sulfoxide extraction. Carbohydr. Res.
- MS Mikkelsen, BM Jesoersen, FH Larsen, A Blennow, SB Engelsen (2013). Molecular structure of large-scale extracted β-glucan from barley and oat: identification of a significantly changed block structure in a high β-glucan barley mutant Food Chem.
- JA Bohn, J BeMiller (1995). (1→3)-β-d-Glucans as biological response modifiers: a review of structure-functional activity relationships Carbohydr. Polym.
- SM Wani, A Gani, SA Mir, FA Masoodi, FA Khanday (2021). β-Glucan: A dual regulator of apoptosis and cell proliferation. International Journal of Biological Macromolecules. 182, 1 July 2021, 1229-1237.
- SM Wani, A Gani, SA Mir, FA Masoodi, FA Khanday (2021). β-Glucan: A dual regulator of apoptosis and cell proliferation, International Journal of Biological Macromolecules, 182, 1229-1237.
- Singla A, Gupta OP, Sagwal V, Kumar A, Patwa N, Mohan N, Ankush, Kumar D, Vir O, Singh J, Kumar L, Lal C, Singh G (2024). Beta-Glucan as a Soluble Dietary Fiber Source: Origins, Biosynthesis, Extraction, Purification, Structural Characteristics, Bioavailability, Biofunctional Attributes, Industrial Utilization, and Global Trade. Nutrients. Mar 21;16(6):900.
- SP Wasser, (2002).Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 60, 258–274.
- V Vetvicka, J Vetvickova (2014). J. Natural immunomodulators and their stimulation of immune reaction: True or false? Anticancer Res. 34, 2275–2282.
- MJ Shear, FC Turner, A Perrault, T Shovelton, (1943). Chemical treatment of tumors. V. Isolation of the hemorrhage-producing fraction from Serratia marcescens (Bacillus prodigiosus) culture filtrate. J. Natl. Cancer Inst. , 4, 81–97.
- P Rathgeb, B Sylven (1954). Fractionation studies on the tumor-necrotizing agent from Serratia marcescens (Shear’s polysaccharide). J. Natl. Cancer Inst. 14, 1099–1108.
- J Yang, J Tu, H Liu, L Wen, Y Jiang, B Yang (2019). Identification of an immunostimulatory polysaccharide in banana. Food Chem. 277, 46–53.
- S Iwanaga, BL Lee (2005). Recent advances in the innate immunity of invertebrate animals. J. Biochem. Mol. Biol. 38, 128–150.;
- S Soltanian, E Stuyven, E Cox, P Sorgeloos, P Bossier (2009) Beta-glucans as immunostimulant in vertebrates and invertebrates. Crit. Rev. Microbiol. 35, 109–138.
- V Vetvicka, L Vannucci, P Sima. J Richter (2019). Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials. Molecules. Mar 30;24(7):1251.
- S Trivedi, K Patel, V Belgamwar, K Wadher (2022). Functional polysaccharide lentinan: Role in anti-cancer therapies and management of carcinomas. Pharmacol. Res.-Mod. Chin. Med. 2, 100045.
- Y Ying, W Hao (2023). Immunomodulatory function and anti-tumor mechanism of natural polysaccharides: A review. Front. Immunol. 14, 1147641.
- Qing-Qing Dong, Qian Wu, Yi Lu, Yi Shi, Ke-Da Yang, Xiao-Ling Xu, Wei Chen (2023). Exploring β-glucan as a micro-nano system for oral delivery targeted the colon, International Journal of Biological Macromolecules, 253, 6.
- Y. Gao, W. Tang, X. Dai, H Gao, G Chen, J Ye, E Chan, HL Koh, X Li, S Zhou (2005). Effects of water-soluble Ganoderma lucidum polysaccharides on the immune functions of patients with advanced lung cancer. J. Med. Food, 8 (2) pp. 159-168.
- H Stier, V Ebbeskotte, J Gruenwald (2014). Immune-modulatory effects of dietary Yeast Beta-1,3/1,6-D-glucan. Nutr. J. 13, 38.
- F Hong, J Yan, JT Baran, DJ Allendorf, RD Hansen, GR Ostroff, PX Xing, N-KV Cheung, GD Ross (2004). Mechanism by which orally administered β-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J. Immunol.173, 797–806.
- Małaczewska, J.; Wojcik, R.; Jung, L.; Siwicki, A.K. Effect of Biolex β-HP on selected parameters of specific and non-specific humoral and cellular immunity in rats. Bull. Vet. Inst. Pulawy 2010, 54, 75–80.
- Xiao, W.; Su, J.; Gao, X.; Yang, H.; Weng, R.; Ni, W.; Gu, Y. The microbiota-gut-brain axis participates in chronic cerebral hypoperfusion by disrupting the metabolism of short-chain fatty acids. Microbiome 2022, 10, 62.
- J Foysal, R Fotedar, MAB Siddik, A Tay (2020). Lactobacillus acidophilus and L. plantarum improve health status, modulate gut microbiota and innate immune response of marron (Cherax cainii). Sci. Rep. 10, 5916.;
- Y Sugiyama, Y Mori, M Nara, Y Kotani, E Nagai, H Kawada, M Kitamura, R Hirano, H Shimokawa, A Nakagawa, et al. (2022) Gut bacterial aromatic amine production: Aromatic amino acid decarboxylase and its effects on peripheral serotonin production. Gut Microbes 14, 2128605.
- EK Mitsou, N Panopoulou, K Turunen, V Spiliotis, A Kyriacou (2010). Prebiotic potential of barley derived β-glucan at low intake levels: A randomised, double-blinded, placebo-controlled clinical study. Food Res. Int. 43, 1086–1092.
- V Vetvicka, B Dvorak, J Vetvickova, et al. (2007). Orally administered marine (1–>3)-beta-d-glucan Phycarine stimulates both humoral and cellular immunity. Int. J. Biol. Macromol., 40 (4), pp. 291-298.
- A Nakashima, K Yamada, O Iwata, et al. (2018). Β-glucan in foods and its physiological functions J. Nutr. Sci. Vitaminol., 64 (1), pp. 8-17.
- LT Tong, K Zhong, L Liu, X Zhou, J Qiu, S Zhou (2015). Effects of dietary hull-less barley β-glucan on the cholesterol metabolism of hypercholesterolemic hamsters. Food Chem., 169, pp. 344-349.
- N Tapola, H Karvonen, L Niskanen, M Mikola, E Sarkkinen (2005). Glycemic responses of oat bran products in type 2 diabetic patients. Nutr. Metab. Cardiovasc. Dis., 15 (4), pp. 255-261.
- TH Gamel, E-SM Abdel-Aal, N Ames, R Duss, SM Tosh (2014). Enzymatic extraction of beta-glucan from oat bran cereals and oat crackers and optimization of viscosity measurement. J. Cereal Sci., 59, pp. 33-40.
- S Chakraborty, VD Rajeswari (2022) Biomedical aspects of beta-glucan on glucose metabolism and its role on primary gene PIK3R1. J. Funct. Foods. 99, 105296.
- PS Thondre, CJK Henry (2009). High-molecular-weight barley β-glucan in chapatis (unleavened Indian flatbread) lowers glycemic index. Nutr. Res. 29, 480–486.
- RV Lobato, VO Silva, EF Andrade, DR Orlando, MG Zangeronimo, RV de Souza, LJ Pereira (2015). Metabolic effects of β-glucans (Saccharomyces cerevisae) per os administration in rats with streptozotocin-induced diabetes. Nutr. Hosp. 32, 256–264.
- EJ Benjamin, P Muntner, A Alonso, MS Bittencourt, CW Callaway, AP Carson, A.M Chamberlain, AR Chang, S Cheng, SR Das, et al. (2019). Heart disease and stroke statistics—2019 update: A report from the American Heart Association. Circulation. 139, e56–e528.
- D Kumar, S Narwal, S Virani, RPS Verma, S Gyawali, GP Singh (2020). Barley grain beta glucan enrichment: Status and opportunities. In Wheat and Barley Grain Biofortification; Gupta, O.P., Pandey, V., Narwal, S., Sharma, P., Ram, S., Singh, G.P., Eds.; Woodhead Publishing: Sawston, UK, pp. 295–308.
- H Zhang, N Zhang, Z Xiong, G Wang, Y Xia, P Lai, L Ai (2018). Structural characterization and rheological properties of β-D-glucan from hull-less barley (Hordeum vulgare L. var. nudum Hook. f.). Phytochemistry. 155, 155–163.
- P Kittisuban, P Ritthiruangdej, M Suphantharika (2014). Optimization of hydroxypropylmethylcellulose, yeast β-glucan, and whey protein levels based on physical properties of gluten-free rice bread using response surface methodology. LWT-Food Sci. Technol. 57, 738–748.
- B Du, Z Bian, B Xu (2014). Skin health promotion effects of natural beta-glucan derived from cereals and microorganisms: A review. Phytother. Res. 28, 159–166.
- H Sung, J Ferlay, RL Siegel, M Laversanne, I Soerjomataram, A Jemal, F Bray (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249.
- V Vetvicka, TV Teplyakova, AB Shintyapina, TA Korolenko (2021). Effects of medicinal fungi-derived β-glucan on tumor progression. J. Fungi. 7, 250.
- J Garcia, F Rodrigues, MJ Saavedra, FM Nunes, G Marques (2022). Bioactive polysaccharides from medicinal mushrooms: A review on their isolation, structural characteristics and antitumor activity. Food Biosci. 49, 101955.
- EJ Murphy, E Rezoagli, I Major, NJ Rowan, JG Laffey (2020). β-glucan metabolic and immunomodulatory properties and potential for clinical application. J. Fungi. 6, 356.;
- A Córdova-Martínez, A Caballero-García, E Roche, DC Noriega (2021). β-Glucans could be adjuvants for SARS-CoV-2 virus vaccines (COVID-19). Int. J. Environ. Res. Public Health. 18, 12636.