Cellular energy-boosting ingredients such as CoQ10, creatine, B vitamins, alpha-lipoic acid, and NAD+ precursors play a vital role in optimizing mitochondrial function and ATP production. As the demand for functional foods and supplements rises, these bioactive compounds are increasingly recognized for enhancing metabolism, reducing fatigue, and supporting healthy aging.
AUTHOR: Daria Šurić, Mpharm, univ.spec.pharm.
Background on cellular energy
Cellular energy, mainly in the form of adenosine triphosphate, is essential for almost all biological processes, from muscle contraction to neuronal signaling1. This crucial molecule is produced through a complex network of metabolic pathways, including glycolysis, the Krebs cycle, and oxidative phosphorylation, all of which depend on a steady supply of specific micronutrients and cofactors2. Functional foods and dietary supplements often incorporate bioactive compounds to support these pathways, thereby improving cellular energy production and overall metabolic efficiency3,4.
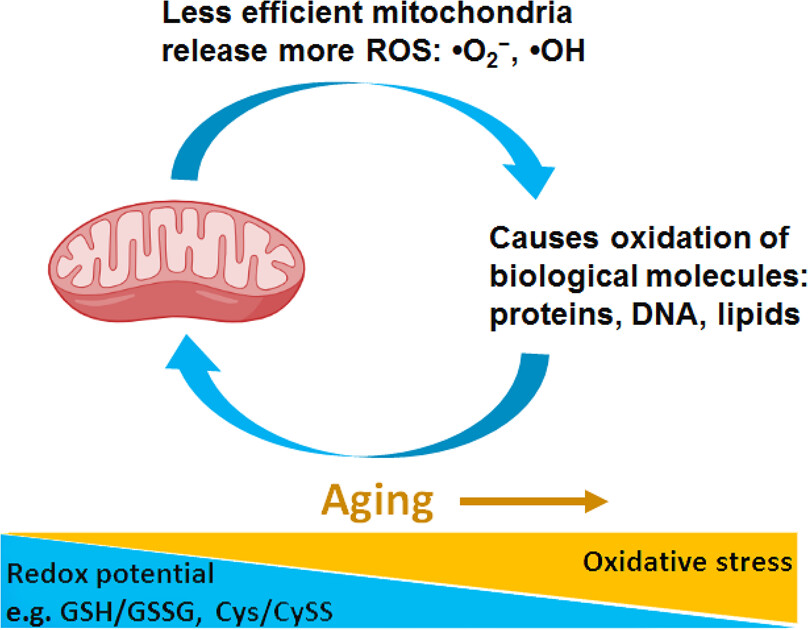
The wide range of ingredients found in these products includes vitamins, minerals, amino acids, botanicals, and other bioactive compounds, each with a unique role in regulating energy metabolism. Mitochondrial dysfunction upon aging results in increased ROS, thus causing enhanced oxidation of biomolecules (proteins, lipids) and opening a positive feedback loop of aging damage (Figure 1).
Figure 1: Schematic presentation of the positive feedback loop between mitochondria dysfunction and oxidative stress upon aging24.
Food supplements containing cellular energy-boosting ingredients
Several essential ingredients in food supplements have shown significant potential to enhance cellular energy production and overall mitochondrial health. These ingredients often operate through various mechanisms, such as increasing substrate availability, optimizing enzyme activity, or strengthening antioxidant defenses to preserve mitochondrial integrity.
For example, compounds like coenzyme Q10 directly participate in the electron transport chain, while others like alpha-lipoic acid serve as powerful antioxidants and cofactors for mitochondrial enzymes. Additional notable supplements include L-carnitine, which helps transport fatty acids into mitochondria, and various NAD+ precursors that elevate cellular levels of this vital coenzyme. The reduction in NAD+ levels, commonly observed in myocardial cells, has been directly linked to insufficient ATP production and a higher risk of conditions such as heart failure, suggesting that NAD+ replenishment could improve energy deficiency in these cases. For instance, coenzyme Q10 plays a crucial role in mitochondrial energy generation and acts as a strong antioxidant, with its natural production decreasing with age, highlighting the need for supplementation to sustain cellular energy and counteract age-related decline.
Ingredients known to boost cellular energy
A diverse array of ingredients, ranging from vitamins and minerals to specialized botanical extracts, has been identified for their potential to enhance cellular energy production through various mechanisms. These ingredients often act as precursors, cofactors, or modulators within the intricate metabolic pathways that generate adenosine triphosphate, the primary energy currency of the cell. The careful regulation of these pathways is essential for maintaining cellular homeostasis and ensuring the optimal functioning of organs and tissues5. Specifically, various macro- and micronutrients are critical for efficient energy metabolism and mitochondrial function, either serving as substrates for catabolism or as vital components of enzymatic systems6.
The growing market for functional foods reflects a global trend toward health consciousness, with consumers increasingly seeking products that offer benefits beyond basic nutritional sustenance. This pursuit often leads to the exploration of dietary interventions and supplementation strategies aimed at optimizing energy metabolism and reducing fatigue, especially during periods of increased physiological demand7.
Coenzyme Q10 (CoQ10)
Coenzyme Q10, a ubiquinone, plays a key role in the mitochondrial electron transport chain as an essential cofactor for several enzymes involved in oxidative phosphorylation, thereby supporting ATP production. It functions as both an electron acceptor and donor, transferring electrons from complex I to complex II and then to complex III, a process crucial for creating the proton gradient that powers ATP synthase9.
Beyond its role in energy production, CoQ10 also acts as a powerful antioxidant, protecting mitochondrial membranes from oxidative damage, which is essential for maintaining metabolic efficiency10. Additionally, CoQ10 regulates cellular signaling and gene expression, affecting pathways related to metabolism and transport by participating in various oxidation-reduction reactions that generate hydrogen peroxide, a secondary messenger that activates transcription factors such as NF-κB.
Creatine
Creatine, an endogenous compound synthesized from amino acids, plays a vital role in rapid ATP regeneration, especially in tissues with high and variable energy demands, such as skeletal muscle and the brain. It functions as a key part of the phosphocreatine system, enabling quick phosphorylation of adenosine diphosphate back to ATP, thus providing an immediate energy reserve during high-intensity, short-duration activities11. Research shows that creatine supplementation not only boosts high-energy phosphate availability but also has antioxidative and neuroprotective effects, helping maintain mitochondrial health under stressful conditions12. This makes it a highly desirable ingredient in dietary supplements designed to enhance athletic performance and support cognitive function. Furthermore, creatine supplementation has been studied for its potential to slow age-related decline in muscle function and overall physical ability by improving mitochondrial health13,14. The ergogenic benefits of creatine monohydrate are well established, enhancing resistance training adaptations and improving performance in short, high-intensity activities15.
B vitamins
The B vitamins, including thiamine (B1), riboflavin (B2), niacin (B3), pantothenic acid (B5), pyridoxine (B6), biotin (B7), folate (B9), and cobalamin (B12), are essential water-soluble micronutrients that act as coenzymes in many metabolic pathways vital for cellular energy production. Although they do not directly provide energy, these vitamins are crucial for transforming macronutrients (carbohydrates, fats, and proteins) into ATP and for serving as important cofactors in enzymatic reactions central to glycolysis, the Krebs cycle, and oxidative phosphorylation. For example, thiamine (B1), riboflavin (B2), and niacin (B3) are especially vital, as they serve as coenzymes in key energy-producing metabolic reactions.

Alpha-lipoic acid (ALA)
Alpha-lipoic acid is a powerful antioxidant and an essential cofactor for mitochondrial enzymes involved in energy metabolism, especially pyruvate dehydrogenase and alpha-ketoglutarate dehydrogenase. This dithiol compound plays a key role in converting pyruvate into acetyl-CoA, a vital step connecting glycolysis to the Krebs cycle, and also takes part in the oxidative decarboxylation of alpha-ketoglutarate within the Krebs cycle. Its unique ability to switch between oxidized and reduced forms enables it to regenerate other antioxidants, such as vitamin C, vitamin E, and glutathione, thereby enhancing its protective effects against oxidative stress, which can damage mitochondrial function. Additionally, alpha-lipoic acid improves glucose uptake and utilization, increasing insulin sensitivity and potentially optimizing substrate availability for ATP production13.
Glutathione
Glutathione, a tripeptide composed of cysteine, glutamate, and glycine, is well known as the body’s main antioxidant, playing a key role in cellular detoxification and redox balance, both of which are closely linked to mitochondrial health and energy production16. Its widespread presence across various cellular compartments enables it to neutralize reactive oxygen species and regenerate other natural antioxidants, helping protect mitochondria from oxidative damage that could otherwise reduce their ability to produce ATP. Besides its direct antioxidant role, glutathione also helps in important detoxification processes, linking with xenobiotics and natural metabolites to promote their removal, preventing their buildup and possible interference with mitochondrial function. Additionally, maintaining healthy glutathione levels is essential for supporting the activity of multiple enzymes involved in mitochondrial respiration and for preventing lipid peroxidation in mitochondrial membranes.
L-carnitine
L-carnitine is an essential quaternary ammonium compound vital for cellular energy metabolism, mainly aiding in the transport of long-chain fatty acids into the mitochondrial matrix for beta-oxidation. This transport process is crucial for producing acetyl-CoA, which then enters the tricarboxylic acid cycle, ultimately generating ATP. In addition to its primary role in fatty acid transport, L-carnitine also has antioxidant properties that help reduce oxidative stress within mitochondria.
NAD
Nicotinamide adenine dinucleotide (NAD+) is a vital coenzyme present in all living cells, essential for fundamental metabolic processes, especially those related to cellular energy production. NAD+ serves as a key electron acceptor in catabolic pathways, oxidizing nutrients like glucose, amino acids, and fatty acids to transfer electrons to NADH and FADH2, which then drive the electron transport chain for ATP production. Beyond its role in electron transfer, NAD+ is also an important substrate for enzymes such as sirtuins and poly-ADP-ribose polymerases, which are essential for DNA repair, gene regulation, and cellular aging, linking cellular energy status to critical regulatory functions. The balance between the oxidized form (NAD+) and the reduced form (NADH) is vital for maintaining metabolic homeostasis, mitochondrial function, and overall cell health17. The NAD+/NADH ratio specifically influences the activity of NAD-linked dehydrogenases, heavily affecting ATP synthesis efficiency.
Supplementing with NAD+ precursors like nicotinamide riboside or nicotinamide mononucleotide can raise cellular NAD+ levels, showing potential in improving mitochondrial performance and reducing age-related metabolic decline in various preclinical models. This strategy aims to enhance the cellular energy environment, potentially increasing resilience to stress and supporting overall health, especially with aging. Such approaches are increasingly being studied for their possible benefits in cardiovascular health and neuroprotection, given NAD+’s fundamental role in energy metabolism and cell regulation. Since NAD+ is central to cellular bioenergetics, its levels and the NAD+/NADH ratio are crucial indicators of cell health and function.
SOD
Superoxide dismutase is a vital metalloenzyme that plays a key role in the cellular antioxidant defense system by catalyzing the dismutation of superoxide radicals into oxygen and hydrogen peroxide, thereby preventing oxidative damage to cellular components including mitochondria. This enzymatic process is essential because superoxide radicals, if left unchecked, can initiate lipid peroxidation and damage DNA and proteins, significantly impairing mitochondrial function and overall cellular energy production. Dysregulation of SOD activity, therefore, can directly lead to mitochondrial dysfunction and decreased ATP generation, emphasizing its importance in maintaining cellular energy balance. Additionally, specific isoforms of SOD, such as mitochondrial superoxide dismutase, are precisely localized to safeguard the primary site of reactive oxygen species production during oxidative phosphorylation, directly affecting the efficiency of ATP synthesis19. Furthermore, the complex regulation of SOD activity is crucial for balancing reactive oxygen species production and neutralization, which in turn influences mitochondrial health and cellular energy output.
Declining NAD+ levels with age impair tissue function, contributing to various age-related diseases, and highlight the potential for NAD+ replenishment strategies to counteract these degenerative conditions. As a result, approaches aimed at restoring NAD+ levels, such as supplementing with its precursors, are actively being studied to improve mitochondrial function and reduce age-related metabolic decline20. The decrease in NAD+ bioavailability, a hallmark of metabolic decline with aging, is closely linked to failing mitochondrial function and significantly contributes to the global burden of cardiovascular diseases.
Multi-ingredient supplements
While single-ingredient supplements target specific pathways, multi-ingredient formulations often provide a synergistic approach by combining various compounds to improve different aspects of cellular energy metabolism and mitochondrial function at the same time21. These formulations usually aim to address multiple bottlenecks in energy production, such as nutrient deficiencies, oxidative stress, and mitochondrial dysfunction, to deliver a more comprehensive therapeutic benefit13. For example, combining NAD+ precursors with antioxidants and mitochondrial support compounds can collectively strengthen cellular resilience against various stressors, thereby boosting energy output. Such all-encompassing supplements are designed to support the complex interaction of cellular processes, from substrate use to ATP synthesis, ensuring ongoing cellular vitality and adaptability.
Dosage and bioavailability considerations
The effectiveness of cellular energy-boosting ingredients in food supplements highly depends on their proper dosage and bioavailability, which determine the actual amount that reaches target cellular areas. Factors such as chemical form, absorption rates, and first-pass metabolism greatly affect how much of a supplement the cells can effectively use for energy production. Additionally, the timing and frequency of intake are crucial in maintaining steady intracellular levels of these compounds, further impacting their therapeutic effects.
Understanding these pharmacokinetic properties is key to maximizing the potential of these supplements in boosting cellular energy and mitochondrial health. This detailed understanding is essential for creating effective supplementation plans that truly enhance cellular bioenergetics and help counteract age-related decline. Therefore, thorough scientific research into the best delivery methods and cellular uptake of these nutrients is vital for improving their use in supporting overall health and fighting chronic diseases.
Potential side effects and interactions
It is essential to consider the potential for adverse effects and interactions with other medications or supplements, particularly given the broad physiological roles of many cellular energy-boosting ingredients. This necessitates a thorough understanding of individual physiological responses and careful monitoring to ensure optimal therapeutic benefit without compromising patient safety. Therefore, comprehensive pharmacological and toxicological assessments are imperative for each ingredient to delineate safe dosage ranges and identify contraindications, ensuring a favorable risk-benefit profile for consumers. Furthermore, emerging research increasingly focuses on personalized nutrition approaches, leveraging genetic and metabolic profiles to tailor supplement regimens for enhanced efficacy and reduced adverse reactions.
This individualized strategy acknowledges the diverse biochemical landscapes of individuals, moving beyond a one-size-fits-all approach to supplementation22. This approach integrates an understanding of how specific nutrients and cofactors, such as NAD+ precursors, influence cellular bioenergetics at an individual level, optimizing interventions for improved mitochondrial function and overall cellular vitality.
Future trends
Future trends in cellular energy-boosting ingredients point towards personalized nutrition approaches, leveraging individual genetic and microbiome profiles to tailor supplement and functional food recommendations for maximal efficacy. This shift will likely involve advanced diagnostics, including metabolomics and genomics, to precisely identify specific energetic deficiencies and personalize interventions. Further research will focus on the intricate interplay between diet, gut microbiota, and host metabolism to unlock more sophisticated and targeted nutritional strategies for optimizing cellular bioenergetics.
Moreover, ongoing investigations are exploring the long-term safety and optimal dosing of these compounds, particularly NAD+ precursors, where uncertainties remain regarding pharmacokinetics and pharmacodynamics. For instance, the global market for nicotinamide mononucleotide, a prominent NAD+ precursor, was valued at US$252.7 million in 2020 and is projected to reach US$385.7 million by 2027, underscoring the substantial commercial interest and investment in this sector23. This substantial growth is primarily driven by an aging global population and increasing health consciousness, which fuels a rising demand for anti-aging and health-promoting products, including cellular energy boosters.
Conclusion
Improving cellular energy production is a key focus in modern nutritional science, as efficient mitochondrial function supports nearly every physiological process – from muscle movement and brain function to immune health and metabolism. The growing research on bioactive compounds such as CoQ10, creatine, alpha-lipoic acid, B vitamins, L-carnitine, glutathione, and NAD+ precursors emphasizes their combined potential to enhance mitochondrial efficiency, reduce oxidative stress, and slow down age-related decline.
However, despite promising evidence, differences in individual metabolic responses, bioavailability, and limited long-term safety data highlight the need for more tailored, personalized supplementation methods. Incorporating omics-based diagnostics – such as metabolomics and nutrigenomics – could enable precision nutrition strategies that maximize mitochondrial energy production tailored to each person’s unique biochemical profile.
In parallel, advances in formulation science and delivery systems (e.g., liposomal encapsulation, sustained-release matrices) are expected to enhance the stability and cellular uptake of these ingredients, boosting their real-world effectiveness. Rigorous clinical studies are crucial to determine optimal dosages, verify synergistic effects among multi-ingredient formulations, and ensure long-term safety.
Given the growing consumer interest in functional foods and nutraceuticals for energy, vitality, and longevity, collaboration among researchers, manufacturers, and healthcare professionals will be essential to turn laboratory discoveries into safe, effective, and evidence-based products. Ongoing innovation and scientific validation will ultimately shape the next generation of cellular energy-boosting supplements, connecting nutrition, preventive medicine, and healthy aging.

References
1.Rutkowska, J., & Pasqualone, A. (2025). Plant Extracts as Functional Food Ingredients. Foods, 14(3), 374. https://doi.org/10.3390/foods14030374
2. Spriet, L. L. (2022). Diet and Nutraceuticals for Mental and Physical Performance in Athletes. Sports Medicine, 52, 1. https://doi.org/10.1007/s40279-022-01794-w
3. Vignesh, A., Amal, T. C., Sarvalingam, A., & Vasanth, K. (2024). A review on the influence of nutraceuticals and functional foods on health [Review of A review on the influence of nutraceuticals and functional foods on health]. Food Chemistry Advances, 5, 100749. Elsevier BV. https://doi.org/10.1016/j.focha.2024.100749
4. Boggia, R., Zunin, P., & Turrini, F. (2020). Functional Foods and Food Supplements. Applied Sciences, 10(23), 8538. https://doi.org/10.3390/app10238538
5. Liu, H., Wang, S., Wang, J., Guo, X., Song, Y., Fu, K., Gao, Z., Liu, D., He, W., & Yang, L. (2025). Energy metabolism in health and diseases [Review of Energy metabolism in health and diseases]. Signal Transduction and Targeted Therapy, 10(1). Springer Nature. https://doi.org/10.1038/s41392-025-02141-x
6. Nieuwenhuizen, A. G., & Schothorst, E. M. van. (2021). Energy Metabolism and Diet. Nutrients, 13(6), 1907. https://doi.org/10.3390/nu13061907
7. Lee, M., Hsu, Y.-J., Shen, S., Ho, C.-S., & Huang, C. (2023). A functional evaluation of anti-fatigue and exercise performance improvement following vitamin B complex supplementation in healthy humans, a randomized double-blind trial. International Journal of Medical Sciences, 20(10), 1272. https://doi.org/10.7150/ijms.86738
8. Yasul, Y., Yılmaz, B., Şenel, Ö., Kurt, D., Akbulut, T., Çalıkuşu, A., Anadol, E., & Yılmaz, C. (2025). Evaluating the impact of coenzyme Q10 and high-intensity interval training on lactate threshold and Plasma blood gases in rats: a randomized controlled trial. European Journal of Applied Physiology. https://doi.org/10.1007/s00421-025-05756-8
9. Spinelli, J. B., & Haigis, M. C. (2018). The multifaceted contributions of mitochondria to cellular metabolism [Review of The multifaceted contributions of mitochondria to cellular metabolism]. Nature Cell Biology, 20(7), 745. Nature Portfolio. https://doi.org/10.1038/s41556-018-0124-1
10. Barnish, M., Sheikh, M., & Scholey, A. (2023). Nutrient Therapy for the Improvement of Fatigue Symptoms [Review of Nutrient Therapy for the Improvement of Fatigue Symptoms]. Nutrients, 15(9), 2154. Multidisciplinary Digital Publishing Institute. https://doi.org/10.3390/nu15092154
11. Alzharani, M. A., Alshuwaier, G. O., Aljaloud, K. S., Al‐Tannak, N. F., & Watson, D. G. (2020). Metabolomics profiling of plasma, urine and saliva after short term training in young professional football players in Saudi Arabia. Scientific Reports, 10(1). https://doi.org/10.1038/s41598-020-75755-6
12. Marshall, R. P., Droste, J.-N., Gießing, J., & Kreider, R. B. (2022). Role of Creatine Supplementation in Conditions Involving Mitochondrial Dysfunction: A Narrative Review [Review of Role of Creatine Supplementation in Conditions Involving Mitochondrial Dysfunction: A Narrative Review]. Nutrients, 14(3), 529. Multidisciplinary Digital Publishing Institute. https://doi.org/10.3390/nu14030529
13. Broome, S. C., Whitfield, J., Karagounis, L. G., & Hawley, J. A. (2024). Mitochondria as Nutritional Targets to Maintain Muscle Health and Physical Function During Ageing [Review of Mitochondria as Nutritional Targets to Maintain Muscle Health and Physical Function During Ageing]. Sports Medicine, 54(9), 2291. Springer Science+Business Media. https://doi.org/10.1007/s40279-024-02072-7
14. Chang, H., & Leem, Y. (2023). The potential role of creatine supplementation in neurodegenerative diseases. Physical Activity and Nutrition, 27(4), 48. https://doi.org/10.20463/pan.2023.0037
15. Forbes, S. C., Candow, D. G., Neto, J. H. F., Kennedy, M. D., Forbes, J. L., Machado, M., Bustillo, E., Gomez-Lopez, J., Zapata, A., & António, J. (2023). Creatine supplementation and endurance performance: surges and sprints to win the race [Review of Creatine supplementation and endurance performance: surges and sprints to win the race]. Journal of the International Society of Sports Nutrition, 20(1). Springer Science+Business Media. https://doi.org/10.1080/15502783.2023.2204071
16. Mantle, D., & Hargreaves, I. P. (2022). Mitochondrial Dysfunction and Neurodegenerative Disorders: Role of Nutritional Supplementation [Review of Mitochondrial Dysfunction and Neurodegenerative Disorders: Role of Nutritional Supplementation]. International Journal of Molecular Sciences, 23(20), 12603. Multidisciplinary Digital Publishing Institute. https://doi.org/10.3390/ijms232012603
17. Yusri, K., Jose, S., Vermeulen, K., Tan, T. C., & Sorrentino, V. (2025). The role of NAD+ metabolism and its modulation of mitochondria in aging and disease [Review of The role of NAD+ metabolism and its modulation of mitochondria in aging and disease]. Npj Metabolic Health and Disease, 3(1). https://doi.org/10.1038/s44324-025-00067-0
18. Zeybel, M., Altay, Ö., Arif, M., Li, X., Yang, H., Fredolini, C., Akyıldız, M., Sağlam, B., Gönenli, M. G., Ural, D., Kim, W., Schwenk, J. M., Zhang, C., Shoaie, S., Nielsen, J., Uhlén, M., Borén, J., & Mardinoğlu, A. (2021). Combined Metabolic Activators Reduces Liver Fat in Nonalcoholic Fatty Liver Disease Patients. medRxiv (Cold Spring Harbor Laboratory). https://doi.org/10.1101/2021.05.20.21257480
19. Li, Y., Tian, X., Luo, J., Bao, T., Wang, S., & Wu, X. (2024). Molecular mechanisms of aging and anti-aging strategies [Review of Molecular mechanisms of aging and anti-aging strategies]. Cell Communication and Signaling, 22(1). BioMed Central. https://doi.org/10.1186/s12964-024-01663-1
20. Marmolejo-Garza, A., Châtre, L., Croteau, D. L., Herron-Bedoya, A., Luu, M. D. A., Bernay, B., Pontin, J., Bohr, V. A., Boddeke, E., & Dolga, A. M. (2024). Nicotinamide riboside modulates the reactive species interactome, bioenergetic status and proteomic landscape in a brain-region-specific manner. Neurobiology of Disease, 200, 106645. https://doi.org/10.1016/j.nbd.2024.106645
21. Neufeld, L. M., Ho, E., Obeid, R., Tzoulis, C., Green, M., Huber, L., Stout, M., & Griffiths, J. C. (2023). Advancing nutrition science to meet evolving global health needs. European Journal of Nutrition, 62, 1. https://doi.org/10.1007/s00394-023-03276-9
22. Tenchov, R., Sasso, J. M., Wang, X., & Zhou, Q. A. (2023). Aging Hallmarks and Progression and Age-Related Diseases: A Landscape View of Research Advancement [Review of Aging Hallmarks and Progression and Age-Related Diseases: A Landscape View of Research Advancement]. ACS Chemical Neuroscience, 15(1), 1. American Chemical Society. https://doi.org/10.1021/acschemneuro.3c00531
23. Song, Q., Zhou, X., Xu, K., Liu, S., Zhu, X., & Yang, J. (2023). The Safety and Antiaging Effects of Nicotinamide Mononucleotide in Human Clinical Trials: an Update [Review of The Safety and Antiaging Effects of Nicotinamide Mononucleotide in Human Clinical Trials: an Update]. Advances in Nutrition, 14(6), 1416. Elsevier BV. https://doi.org/10.1016/j.advnut.2023.08.008
24. Tenchov, Rumiana, Janet M. Sasso, Xinmei Wang, and Qiongqiong Angela Zhou.
“Aging Hallmarks and Progression and Age-Related Diseases: A Landscape View of Research Advancement.” ACS Chemical Neuroscience 15, no. 1 (2024): 1–30. https://doi.org/10.1021/acschemneuro.3c00531




